the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Effect of legume intercropping on N2O emissions and CH4 uptake during maize production in the Great Rift Valley, Ethiopia
Shimelis Gizachew Raji
Intercropping with legumes is an important component of climate-smart agriculture (CSA) in sub-Saharan Africa, but little is known about its effect on soil greenhouse gas (GHG) exchange. A field experiment was established at Hawassa in the Ethiopian rift valley, comparing nitrous oxide (N2O) and methane (CH4) fluxes in minerally fertilized maize (64 kg N ha−1) with and without Crotalaria (C. juncea) or lablab (L. purpureus) as intercrops over two growing seasons. To study the effect of intercropping time, intercrops were sown either 3 or 6 weeks after maize. The legumes were harvested at flowering, and half of the aboveground biomass was mulched. In the first season, cumulative N2O emissions were largest in 3-week lablab, with all other treatments being equal to or lower than the fertilized maize mono-crop. After reducing mineral N input to intercropped systems by 50 % in the second season, N2O emissions were comparable with the fully fertilized control. Maize-yield-scaled N2O emissions in the first season increased linearly with aboveground legume N yield (p=0.01), but not in the second season when early rains resulted in less legume biomass because of shading by maize. Growing-season N2O-N emission factors varied from 0.02 % to 0.25 % in 2015 and 0.11 % to 0.20 % in 2016 of the estimated total N input. Growing-season CH4 uptake ranged from 1.0 to 1.5 kg CH4-C ha−1, with no significant differences between treatments or years but setting off the N2O-associated emissions by up to 69 %. Our results suggest that leguminous intercrops may increase N2O emissions when developing large biomass in dry years but, when mulched, can replace part of the fertilizer N in normal years, thus supporting CSA goals while intensifying crop production in the region.
- Article
(1349 KB) -
Supplement
(266 KB) - BibTeX
- EndNote
With a rapidly increasing population and declining agricultural land in sub-Saharan Africa (SSA), increasing productivity per area (intensification) is the only viable alternative for producing sufficient food and feed (Hickman et al., 2014a). Intensification entails the increased use of inorganic fertilizers, which may cause emissions of nitrous oxide (N2O). Abundant ammonium () may also reduce the soil CH4 sink by competing with CH4 for the active binding site of methane monooxygenase, the key enzyme of CH4 oxidation (Bédard and Knowles, 1989). Climate-smart agriculture (CSA) is an approach to transform agricultural practices in a changing climate with the triple objective of increasing agricultural productivity, building climate resilience and reducing greenhouse gas (GHG) emissions (Neufeldt et al., 2013). Potential CSA practices include improved water management, use of improved livestock and crop species, conservation farming, agroforestry, and crop diversification as well as improved soil fertility management practices (Makate et al., 2019). Legume intercropping is one way to diversify and intensify cropping systems while contributing to the food and nutritional security of smallholder farmers (de Jager et al., 2019). Legume intercropping can also be used to add biologically fixed nitrogen to soils, build soil carbon and improve soil quality (Bedoussac et al., 2015). As such, it is a powerful approach to reduce greenhouse gas emissions by replacing inorganic fertilizers and GHG emissions associated with their production. However, GHG measurements in SSA crop production systems in general, and in legume intercropping systems in particular, are scarce and proof of concept for the mitigation potential of legume intercropping is missing (Kim et al., 2016; Hickman et al., 2014b). Moreover, modeling studies predict significant negative impacts of climate change on crop productivity in Africa (Blanc and Strobl, 2013) and it is largely unknown how these and the countermeasures taken to maintain agricultural productivity will affect GHG emissions.
Crop production is a major source of N2O, the third-most important anthropogenic GHG after CH4 and CO2 (IPCC, 2014). Emission rates of N2O reported for SSA crop production so far are low (Kim et al., 2016) owing to low fertilization rates, but they may increase with increasing intensification. Inorganic and organic N added to soil provide ammonium () and nitrate () for nitrification and denitrification, respectively, which are the two main processes of microbial N2O production in soil (Khalil et al., 2004). The rate of N2O formation depends greatly on the extent and distribution of anoxic microsites in soils, which is controlled by moisture, texture and the distribution of decomposable organic matter and fueling heterotrophic and autotrophic respiration, respectively (Schlüter et al., 2019; Wrage-Mönnig et al., 2018). The magnitude of soil N2O emissions depends on O2 availability as controlled by soil moisture and respiration, the availability of mineral N and readily decomposable C (Harrison-Kirk et al., 2013), and soil pH (Russenes et al., 2016), all of which are affected by management practices. Other important factors are soil type (Davidson et al., 2000) and temperature (Schaufler et al., 2010). The N2O yield of nitrification and the production and reduction of N2O during denitrification are further controlled by soil pH (Bakken et al., 2012; Nadeem et al., 2019) and by the balance between oxidizable carbon and available (Wu et al., 2018). Mulching and the incorporation of crop residues lead to increased N mineralization and respiratory O2 consumption, thus potentially enhancing N2O emissions both from nitrification and denitrification (Drury et al., 1991) if soil moisture is sufficient to support microbial activity and restrict O2 diffusion into the soil. Accordingly, N2O emissions are variable in time, often following rainfall events (Schwenke et al., 2016).
Crop diversification by combining legumes with cereals, both in rotation and intercropping, enhances overall productivity and resource use efficiency if managed properly (Ehrmann and Ritz, 2014). Intercropping maize with grain legumes is common in the Great Rift Valley of Ethiopia and a central component in CSA (Arslan et al., 2015). In low-input systems common to the Great Rift Valley, the integration of legumes with cereals diversifies the produce and improves farm income and nutritional diversity for smallholder farmers (Sime and Aune, 2018). Moreover, by partially replacing energy-intensive synthetic N, intercropping with legumes may increase the sustainability of the agroecosystem as a whole (Carranca et al., 2015). However, to make the best use of the resource complementarity of intercrops and main crops, the planting time of the intercrop has to be optimized so that the maximum nutrient demand of the two components occurs at different times (Carruthers et al., 2000). The timing of intercrops could also affect N2O emissions if N mineralization from legume residues is poorly synchronized with the N requirement of the cereal crop. This can be counteracted by reducing mineral N additions to intercropping systems, but the timing of the intercrop (sowing date relative to the cereal crop) remains an issue that has, to the best of our knowledge, not been studied with regard to N2O emissions.
Intercropping and mulching may also affect the soil's capacity to oxidize atmospheric CH4 as abundant might inhibit methanotrophs (Laanbroek and Bodelier, 2004). However, field studies with the incorporation of leguminous or non-leguminous catch crops have been inconclusive (e.g., Sanz-Cobena et al., 2014). In a meta-study on CH4 fluxes in non-wetland soils, Aronson and Helliker (2010) concluded that N inhibition of CH4 uptake is unlikely at fertilization rates below 100 kg N ha−1 yr−1 and that, much to the contrary, N addition may stimulate CH4 uptake in N-limited soils. Ho et al. (2015) found that the incorporation of organic residues stimulated CH4 uptake even in fairly N-rich Dutch soils. Apart from providing reactive nitrogen to the soil, leguminous intercrops may also affect CH4 uptake by lowering soil moisture and thus increasing the diffusive flux of atmospheric CH4 into the soil. For instance, Wanyama et al. (2019) found that CH4 uptake in soil was negatively correlated with mean annual water-filled pore space in a study on different land use intensities in Kenya.
In a review on N2O fluxes in agricultural legume crops, Rochette and Janzen (2005) concluded that the effect of legumes on N2O emissions is to be attributed to the release of extra N by the rhizodeposition of soluble N compounds and the decomposition of nodules rather than to the process of nitrogen fixation itself. Intercropped legumes may thus affect N2O emissions in two ways: by directly providing organic N or by modulating the competition between plants and microbes for soil N, for example by acting as an additional N sink prior to nodulation. Compared to mineral fertilizers, N supply from biological fixation is considered environmentally friendly as it can potentially replace industrially fixed N (Jensen and Hauggaard-Nielsen, 2003), provided that crop yields remain the same. However, combining easily degradable crop residues with synthetic N can lead to elevated N2O emissions (Baggs et al., 2000), potentially compromising the environmental friendliness of intercropping in CSA. It is well known that the effect of crop residues on N2O emission depends on a variety of factors such as residue amount and quality (C : N ratio, lignin and cellulose content), soil properties (e.g., texture), placement mode (mulching, incorporation), and soil moisture and temperature regimes (Sanz-Cobena et al., 2014; Li et al., 2016). So far, only a limited number of studies address the effect of legume intercropping on N2O emissions and CH4 uptake in SSA crop production (Baggs et al., 2006; Millar et al., 2004; Dick et al., 2008).
The main objective of the present study was to evaluate the effects of forage legume intercropping with maize on N2O and CH4 emissions during maize production in the Ethiopian Great Rift Valley. We hypothesized that forage legumes increase N2O emissions and decrease CH4 uptake depending on aboveground biomass, legume species and sowing date; legumes intercropped 3 weeks after sowing maize would result in higher yields than those intercropped 6 weeks after maize and lead to increased N2O emissions if used with full-dose mineral fertilization. With late intercropping, legume yields would be suppressed, having little to no effect on N2O emissions. Hence, choosing legume species and the sowing date as well as accounting for potential N inputs from legume intercrops could allow for a better management of legume intercropping in SSA with reduced GHG emissions.
2.1 Study area
The field experiment was conducted during 2 years (2015–2016) at the Hawassa University Research Farm (7∘3′3.4′′ N, 38∘30′20.4′′ E) at an altitude of 1660 m a.s.l. The mean annual rainfall is 961 mm, with a bimodal pattern. The rainy season between June and October accounts for close to 80 % of the annual rainfall. Average maximum and minimum monthly temperatures are 27.4 and 12.9 ∘C, respectively. The soil is clay–loam (46 % sand, 26 % silt, 28 % clay) derived from weathered volcanic rock (Andosols), with a bulk density of 1.25±0.05 g cm−3, a total N content of 0.12 %, an organic C content of 1.64 %, an available Olsen P content of 175 mg kg−1 and a pH of 6.14.
2.2 Experimental design and treatments
Experimental plots (20 m2) were laid out in a completely randomized block design (RCBD) with four replicates and six treatments: unfertilized maize mono-crop (M-F), fertilized maize mono-crop (M + F), Crotalaria intercropping 3 (M + Cr3w) and 6 (M + Cr6w) weeks after sowing maize, and lablab intercropping 3 (M + Lb3w) and 6 (M + Lb6w) weeks after sowing maize (Table 2). The seed bed was prepared in both years by a mold board plow to a depth of 0.25 m followed by harrowing by a tractor. A hybrid maize variety, BH-540 (released in 1995), was sown on 30 May 2015 and 7 May 2016. Maize was planted at a density of 53 333 plants ha−1. Following national fertilization recommendations, diammonium phosphate (18 kg N, 20 kg P) was applied manually at planting and urea (46 kg N) was applied 4 weeks after sowing maize to all treatments except for the unfertilized control. The N fertilization rate was halved for the intercropping treatments in the 2016 season to account for carryover of N from forage legumes grown in the previous year. The forage legumes Crotalaria (C. juncea) and lablab (L. purpureus) were planted between maize rows at a density of 500 000 and 250 000 plants ha−1, respectively.
The aboveground forage legume biomass was harvested at flowering, and half of it was removed. The remaining half was spread manually between the maize rows after cutting the fresh biomass into ∼10 cm pieces. The 3- and 6-week intercrops were mulched on 27 July and 4 September 2015 and 2 August and 8 September 2016. As the mulching was done plot-wise, plots within the same treatment received different amounts of mulch depending on the legume yield of each plot. In the 2016 growing season, all treatments were kept on the same plots as in 2015, capitalizing on plot-specific N and C input from previous mulch. Aboveground dry matter yield was determined by drying a subsample at 72 ∘C for 48 h, and C and N contents were measured by an element analyzer.
2.3 N2O and CH4 fluxes and ancillary data
GHG exchange was monitored weekly at different spots within the middle maize row by static, non-vented chambers (Rochette and Eriksen-Hamel, 2008). We used custom-made aluminum chambers with an internal volume of 0.144 m3 and a cross-sectional area of 0.36 m2 (Fig. S1 in the Supplement). The chambers were pushed gently ∼3 cm into the soil, including two to five legume plants in the headspace. The septum was left open during deployment; once the chamber was inserted into the soil, the septum was closed and the base of the chamber was sealed around the circumference using moist clay.
Sampling was carried out weekly during the period June to September 2015 and May to September 2016 on 15 and 17 sampling dates, respectively. Gas samples were collected between 09:00 and 14:00 EAT (UTC+3). For each flux estimate, four gas samples were drawn from the chamber headspace at 15 min intervals, starting immediately after deployment. Samples were taken with a 20 mL polypropylene syringe equipped with a three-way valve. Before transferring the sample to a pre-evacuated 10 cc serum vial crimp-sealed with butyl septa, the sample was pumped five times in and out of the chamber to obtain a representative sample. Overpressure in the septum vials was maintained to protect the sample from atmospheric contamination during storage and shipment to the Norwegian University of Life Sciences, where the samples were analyzed by gas chromatography. Helium-filled blank vials were included to evaluate contamination, which was found to be less than 3 % of ambient.
All samples were analyzed on a gas chromatograph (GC; model 7890A, Agilent, Santa Clara, CA, USA) connected to an auto-sampler (GC-Pal, CTC, Switzerland). Upon piercing the septum with a hypodermic needle, ca. 1 mL of sample is transported via a peristaltic pump (Gilson minipuls 3, Middleton, W1, USA) to the GC's injection system before reverting the pump to back-flush the injection system. The GC is configured with a Poraplot U wide-bore capillary column connected to a thermal conductivity, flame ionization and electron capture detector (ECD) to analyze CO2, CH4 and N2O, respectively. Helium 5.0 was used as a carrier and Ar∕CH4 (90 : 10 vol ∕ vol) as a makeup gas for the ECD. For calibration, two certified gas mixtures of CO2, N2O and CH4 in helium 5.0 (Linde-AGA, Oslo, Norway), one at ambient concentrations and one ca. 3 times above ambient, were used. A running standard (every tenth sample) was used to evaluate drift of the ECD signal. Emission (CO2, N2O) and uptake (CH4) rates were estimated by fitting linear or quadratic functions to the observed concentration change in the chamber headspace and converting them to area flux according to Eq. (1):
where FGHG is the flux (µg N2O-N m−2 h−1 in the case of N2O; µg CH4-C in the case of CH4), the rate of change in concentration over time (ppm min−1), Vc the volume of the chamber (m3), A the area covered by the chamber (m2), Mn the molar mass of the element in question (g mol−1) and Vn the molecular volume of gas at chamber temperature (m3 mol−1). A quadratic fit was only used in cases in which N2O accumulation in the chamber showed a convex downwards and CH4 uptake a convex upwards trend (i.e., decreasing emission or uptake rates with time) to estimate time-zero rates. R2 values for fluxes >3 µg N2O-N or CH4-C m−2 h−1 were generally ≥0.85; fluxes <3 µg had lower R2 values in some cases but were still included to capture periods with low flux activity. Fluxes were cumulated plot-wise by linear interpolation for each growing season.
In 2016, soil moisture and temperature at 5 cm of depth were monitored hourly using data loggers (Decagon EM50, Pullman, WA, USA) together with ECH2O sensors (Decagon) for volumetric soil water content (VSWC) and temperature at five points across the experimental field. The sensors were placed in the experimental field at five random spots. No data are available for the 2015 season due to equipment failure.
Soil bulk density was measured at 10 random spots in the experimental field using 100 cm3 steel cylinders and drying them at 105 ∘C for 24 h. To calculate daily water-filled pore space values for the 2016 growing season, a particle density of 2.65 g cm−3 was assumed:
where WFPS is the water-filled pore space, VSWC the volumetric soil water content, BD the bulk density and PD the particle density. Daily rainfall data were collected using an on-site rain gauge.
2.4 Estimating N inputs and N2O emission factors
N input from forage legume crop residues was estimated from measured aboveground dry matter yield, its N content and the amount of mulch applied. To account for belowground inputs a shoot-to-root ratio of 2 was assumed for both Crotalaria and lablab (Fageria et al., 2014). Dry matter yields of forage legumes differed greatly depending on sowing time, with yields generally larger in the 3-week than in the 6-week intercropping. Also, forage legumes sown 3 weeks after maize grew faster and were harvested and mulched earlier than those sown 6 weeks after maize. We assumed that 50 % of the legume N (mulched and belowground) was released during the growing season but reduced this amount to 30 % for the aboveground component (mulch) of the 6-week treatments to account for the later mulching date. The proportions becoming available during the growing seasons are conservative estimates based on Odhiambo (2010), who reported that about 50 % of N contained in Crotalaria, lablab and Mucuna was released during a 16-week incubation experiment at optimal temperature and moisture conditions. Placing litter bags into dry surface soil, Abera et al. (2014) found that legume residues decomposed rapidly under in situ conditions in the Ethiopian Great Rift Valley, releasing up to 89 % of the added N within 6 months.
For the second year, 50 % of the N left after the growing season (belowground and aboveground) was assumed to become available, on top of the N input from the newly sown forage legumes. Dry matter yields of forage legumes and estimated N input for the 2 years are presented in Table 1.
Table 1N inputs from forage legumes and fertilization per treatment. Shown are mean values (n=4 ± standard error).
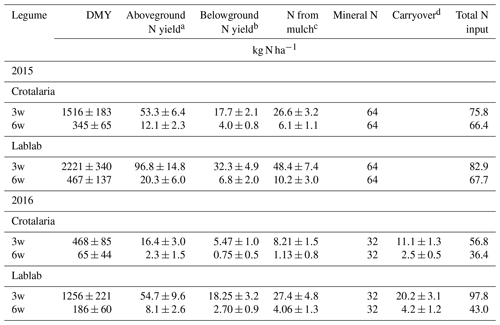
a N content of Crotalaria and lablab was 3.51 and 4.36 %, respectively, measured in two representative samples. DMY: dry matter yield. b Assuming a shoot-to-root ratio of 2 and an average belowground N input from the standing legumes of 50 % during the growing season. c Returning half of the aboveground yield as mulch; assuming an average N release of 50 % and 30 % for 3-week and 6-week treatments, respectively, during the growing season. d Assuming that 50 % of the remaining N becomes available in the following cropping season.
Treatment-specific, growing-season N2O emission factors were calculated as
where EF is the N2O emission factor (% of N input lost as N2O-N), N2Otreatment the cumulative N2O-N emission (from sowing to harvest) in the fertilized and intercropped treatments, N2Ocontrol the emission from the M-F treatment (background emission) and Ninput the estimated total input of N.
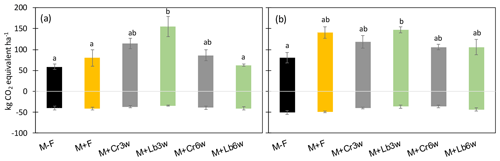
Non-CO2 GHG emissions were calculated as CO2 equivalents, balancing the cumulative seasonal N2O-N emissions with CH4 uptake on the plot level and averaging them for treatments (Table 2, Fig. 5).
Table 2Grain yields, growing-season N2O emission factors, and non-CO2 GHG emissions associated with N2O, CH4, and N2O emission intensities for fertilized treatments with and without legume intercropping during 107 d in 2015 and 123 d in 2016. N input was estimated as outlined in Table 1. Shown are mean values (n=4 ± standard error). Different letters indicate statistical differences at p<0.05.
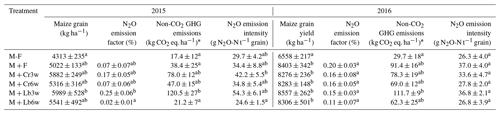
* N2O: 300 CO2 eq.; CH4: 25 CO2 eq.
2.5 Grain yields and yield-scaled N2O emissions
Maize grain yield was determined by manually harvesting the three middle rows (to avoid border effects) of each plot and was standardized to 12.5 % moisture content using a digital grain moisture meter. All values were extrapolated from the plot to the hectare. To estimate yield-scaled N2O emissions (g N2O-N t−1 grain yield), cumulative emissions were divided by grain yield.
2.6 Statistical analysis
Differences in cumulative CH4 and N2O emissions between treatments in each cropping season were tested by analysis of variance (ANOVA) with least significant difference (LSD) used for mean separation after testing the data for normality and homoscedasticity. Cumulative seasonal N2O emissions for 2015 were log-transformed. Statistical significance was declared at p≤0.05.
3.1 Weather conditions
The year 2015 was one of the most severe drought years in decades and, as a result, sowing in 2015 was delayed by 3 weeks compared to 2016. Rain fell late during the growing season, and the cumulative rainfall for April to October was about 100 mm lower in 2015 than in 2016 (Fig. 1d, g).
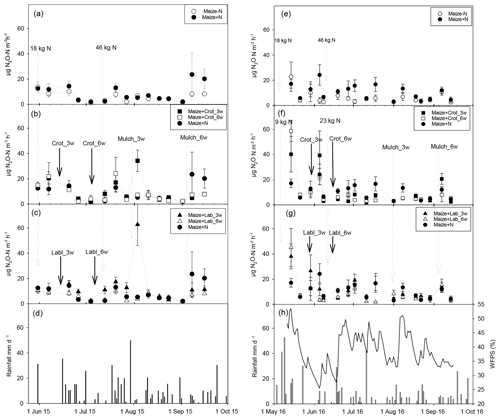
Figure 1Mean N2O emission rates (n=4; error bars: standard error of the mean – SEM) in 2015 (left column) and 2016 (right column), with daily rainfall and water-filled pore space (in 2016 only). Panels (a) and (e) show emission rates in the absence of intercrops, panels (b) and (f) with Crotalaria, and panels (c) and (g) with lablab intercrops.
3.2 N2O fluxes
N2O emission rates in 2015 (treatment means, n=4) ranged from 1.1 to 13.7 µg N m−2 h−1 for the control treatment (Fig. 1a). Similarly, for fertilized maize, N2O emissions ranged from 2 to 23.5 µg N m−2 h−1. Emission fluxes were generally larger for the 3-week intercropping treatments; the 3-week Crotalaria treatment emitted N2O at rates of 1.7–34.3 and the 3-week maize–lablab emitted 1.9–62.7 µg N m−2 h−1, whereas the 6-week maize–Crotalaria emitted 2.1–24.2 µg N m−2 h−1 and the corresponding rate for the 6-week maize–lablab intercrop was 1.5–10.7 µg N m−2 h−1. The generally low emission rates in the 6-week lablab intercropping systems corresponded to poor growth of lablab due to shading by the maize plants. Irrespective of legume species, the highest emission rates were found for intercrops planted 3 weeks after maize (Fig. 1b, c). A peak in N2O emissions occurred in the 3-week intercropping systems around mid-August 2015, which was significantly larger than in the unfertilized control (p=0.013), the fertilized maize mono-crop (p=0.001), and the 6-week Crotalaria (p=0.021) and lablab (p=0.002) intercrops.
During the 2016 season, N2O emission rates in the M-F treatment (unfertilized control) varied between 2.5 and 22.8 µg N m−2 h−1, peaking at the beginning of the season when WFPS was >50 %. There were no significant differences in WFPS values between treatments (data not shown). Fertilized maize had similar rates (3.1–24.2 µg N m−2 h−1), peaking at around 4 weeks after planting. Maize–forage legume treatments had larger emission rates, ranging from 1.8 to 40.2 for 3-week Crotalaria, 3.2 to 58.6 µg N m−2 h−1 for 6-week Crotalaria, 3.9 to 38.0 for 3-week lablab and 1.9 to 45.2 µg N m−2 h−1 for 6-week lablab. In general, emission rates were higher at the beginning than at the end of the cropping season (Fig. 1e–h). Despite higher fluxes for intercropping treatments than in the unfertilized control in week 1 (p=0.162) and 4 (p=0.061), there were no statistically significant differences in flux rates between the treatments.
3.3 Cumulative N2O emissions
During the 2015 growing season, all treatments had equal or higher cumulative N2O emissions than the unfertilized control, with the 3-week lablab intercropping system emitting significantly more N2O than the unfertilized control (p=0.006) and the 6-week lablab intercrop (Fig. 2a). Comparing intercropping treatments with the fertilized control, lablab sown 3 weeks after maize clearly increased N2O emissions but not significantly (p=0.35), whereas all other intercropping treatments had cumulative N2O emissions comparable with the fertilized maize control. Regarding sowing date, 3-week lablab had significantly higher N2O emissions (p<0.01) than its 6-week counterpart, whereas no such effect was seen for Crotalaria.
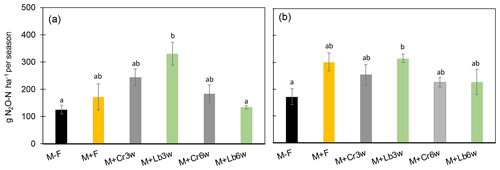
Figure 2Cumulative seasonal N2O-N (g N ha−1 per season) in 2015 (a) and 2016 (b) throughout 107 and 123 d, respectively, in treatments with and without legume intercropping. Error bars denote SEM (n=4). Different letters indicate significant differences at p<0.05. M + F: fertilized maize; M + Cr3w: fertilized maize with Crotalaria sown 3 weeks after maize; M + Cr6w: fertilized maize with Crotalaria sown 6 weeks after maize; M + Lb3w: fertilized maize with lablab sown 3 weeks after maize; M + Lb6w: fertilized maize with lablab sown 6 weeks after maize.
During the 2016 growing season, lablab intercropping 3 weeks after maize showed significantly higher (p<0.01) cumulative N2O emissions than the unfertilized control, but there was no difference between the fully fertilized maize mono-crop and intercropped maize treatments fertilized with 50 % of the mineral N applied in 2015, nor was there any effect of intercropping date (3 vs. 6 weeks; Fig. 2b).
3.4 Legume and maize yields
Aboveground yields of lablab were generally higher than those of Crotalaria (Table 1). Intercropping 3 weeks after maize resulted in higher biomass yields compared to 6 weeks for both legume species. Both legumes grew poorly during the second growing season, particularly Crotalaria. Maize grain yields differed greatly between the years and were roughly 20 % higher in the wetter year of 2016 (Table 2). Better growth conditions for maize in the second year resulted in smaller yields of intercrop legumes.
3.5 N2O emission factor and intensity
Growing-season emission factors (EF) varied from 0.02 % to 0.25 % in 2015 and 0.11 % to 0.20 % in 2016 (Table 2). Of the intercropped treatments, lablab intercropped 3 weeks after maize resulted in a significantly larger emission factor than fertilized maize and other intercropping treatments, whereas there was no significant difference in 2016. Overall, growing-season N2O emission factors were ∼40 % higher in 2016 than in 2015, which is mainly due to the smaller N input in 2016, which was 25 % to 45 % lower than in 2015, except for the 3-week lablab system, which had an estimated 18 % higher N input in 2016 than 2015 (Table 1). The latter was due to the extraordinary high lablab yield in the previous year and its stipulated carryover (Table 1).
Mean yield-scaled N2O emissions in 2015 varied between 25 and 55 g N2O t−1 grain yields. In 2015, 3-week lablab had a higher N2O intensity than 6-week lablab, whereas all other differences were insignificant. In 2016, with the mineral N fertilization reduced to 50 %, N2O emission intensities varied from 26 to 37 g N2O t−1 grain, with no significant effect of legume species, sowing date or N fertilization (Table 2).
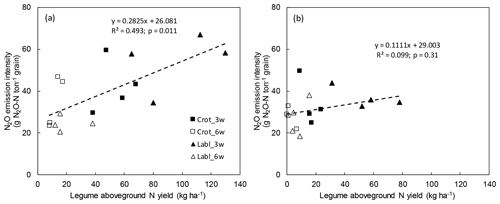
To further explore the variability of N2O emissions, we plotted cumulative N2O emissions plot-wise against legume N yield but found no relationship (not shown). However, when plotting yield-scaled N2O emissions over legume N yield, a significant positive relationship (p=0.01) emerged for 2015 but not 2016 (Fig. 3a, b), suggesting that leguminous N input increased N2O emissions more than maize yields in the dry year of 2015.
3.6 CH4 fluxes
All treatments acted as a net sink for CH4, with uptake rates ranging from 31 to 93 µg C m−2 h−1 in 2015 (Fig. 4a–c). Uptake rates in 2015 were rather constant in time, with somewhat elevated uptake rates towards the end of the season. There were no obvious treatment effects. By contrast, in the wetter year of 2016, CH4 uptake showed a pronounced maximum at the beginning of June with uptake rates of up to 140 µg C m−1 h−1 irrespective of treatment (Fig. 4e–g), when WFPS values declined to below 25 % (Fig. 4h). Methane uptake during this period tended to be greatest in the unfertilized control, while intercropping treatments had smaller uptake rates, but these were not significantly different from maize mono-crop treatments. Differences between treatments on single sampling dates were insignificant throughout the season. The highest CH4 uptake in 2016 was recorded with the lowest WFPS (∼10 %).
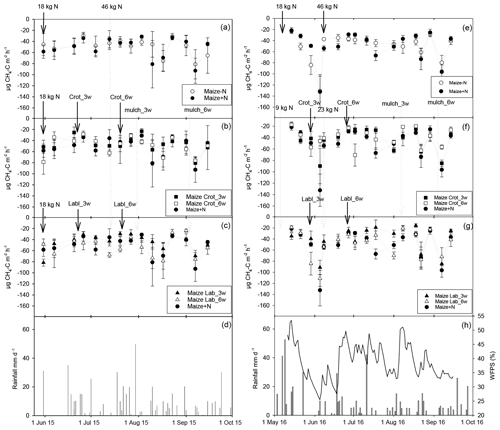
Figure 4Mean CH4 flux in 2015 (left column) and 2016 (right column), with daily rainfall and water-filled pore space (in 2016 only). Error bars show the standard error of the mean (n=4). Panels (a) and (e) show emission rates in the absence of intercrops, panels (b) and (f) with Crotalaria, and panels (c) and (g) with lablab intercropping.
3.7 Cumulative CH4 uptake
Cropping-season cumulative CH4 uptake exceeded 1 kg C ha−1 in both years with no significant effect of intercropping, legume species or time of intercropping (Fig. S2a, b). Maize intercropped with Crotalaria tended to take up less CH4, but this effect was not statistically significant in 2015 or 2016 (p=0.056). Plotting cumulative CH4 uptake plot-wise over legume dry matter yield did not result in a significant relationship, but the highest seasonal uptake rates occurred in plots with the lowest legume dry matter yield (data not shown).
3.8 Total non-CO2 GHG emissions
The relative contribution of CH4 to the non-CO2 GHG emissions of the different cropping systems varied between 22 % and 69 % and was the highest in the non-fertilized maize mono-crop. The 3-week lablab intercropping resulted in significantly higher total emissions compared with 6-week lablab intercropping and maize mono-cropping (Table 2). By contrast, in 2016, legume species but not intercropping time affected the non-CO2 GHG emission balance (p<0.05). Lablab intercropped 3 weeks after maize resulted in significantly higher (p<0.05) total GHG emissions than the unfertilized control but was indistinctive from the fertilized maize mono-crop or other intercrop treatments (Table 2, Fig. 5a, b).
4.1 Maize–legume intercropping and N2O emissions
Background N2O emissions (in unfertilized maize mono-crop) fluctuated between 1.1 and 23.0 µg N2O-N m−2 h−1, which is in the range of previously reported emission rates for soils in SSA with low N fertilizer input (0–20 µg N2O-N m−2 h−1; Pelster et al., 2017). Baseline emissions were somewhat higher in the wetter season of 2016 owing to ∼100 mm more rainfall at the beginning of the season (Fig. 1d, g). Elevated emission rates >30 µg N2O-N m−2 h−1 occurred in 2015 on a few occasions in intercrop treatments, notably in mid-August when rain fell right after mulching of the 3-week intercrops. Mulching of the 6-week intercrops did not affect N2O emissions, probably because the mulched legume biomass was too small to affect the flux (Fig. 1b, c; Table 1). In 2016, mulching of the 3-week legumes was followed by rainfall, increasing the WFPS to 50 % (Fig. 1h) but without resulting in elevated N2O emission rates (Fig. 1f, g). Together, this suggests that the direct effect of mulching on N2O emissions is highly dependent on soil moisture and the amount of mulch and cannot be generalized, contrary to our hypothesis that legume intercrops would invariably increase N2O emissions.
Legume dry matter yields varied strongly (100 to 3000 kg ha−1) throughout the two experimental years (Table 1, Fig. 3) depending on species, intercropping time and weather. Lablab grew more vigorously and realized larger dry matter yields than Crotalaria (Table 1). Moreover, lablab is known to be a better N2 fixer than Crotalaria (Ojiem et al., 2007), presumably leading to higher N input, which would explain larger N2O emissions with this intercrop (Fig. 2). The 3-week intercrops performed generally better than the 6-week intercrops. This was particularly apparent for the low-growing lablab (Table 1). Weather at the beginning of the season played a major role for the growth performance of the intercrops by controlling maize growth, which in turn controlled legume growth by shading. Together, this resulted in a wide range of potential leguminous N inputs in our experiment, which could be used to examine their overall effect on N2O emissions on a seasonal basis under the semiarid conditions of the central Ethiopian rift valley. Surprisingly, we did not find any significant relationship between estimated total N input or legume N yield and cumulative N2O emissions. This may be due to the notoriously high spatial and temporal variability of N2O emission rates (Flessa et al., 1995) or reflect the fact that intercropping had no effect or opposing effects on N2O-forming processes. Cumulative N2O emissions and legume N yields integrate over the entire season and do not capture the seasonal dynamics of soil N cycling and N uptake, which could obscure or cancel out transient legume effects on N2O emissions. Possibly, N released in intercropping treatments was efficiently absorbed by the main crop, even though intercropping did not lead to significantly higher maize grain yields in our experiment. Alternatively, changes in physicochemical conditions brought about by intercrops, such as potentially lower soil moisture due to more evapotranspiration, may have counteracted the commonly observed stimulating effect of legume N on N2O emissions (Almaraz et al., 2009; Sant'Anna et al., 2018).
We found a significant positive relationship between N2O intensity and legume N yields in 2015, suggesting that intercropped legumes indeed increase N2O emissions relative to maize yields (Fig. 3a). It is impossible to say, however, whether this relationship was driven by the extra N entering the system through biological N fixation or whether an increasing legume biomass affected physicochemical conditions in the rhizosphere favoring N2O formation. In 2016, legume dry matter yields were much lower than in 2015 owing to early rains favoring maize growth, and no significant relationship with N2O intensity was found (Fig. 3b). This illustrates that the effect of legume intercropping on N2O emissions is highly dependent on sowing date and weather, both of which control the growth of legume and main crops and ultimately the amount and fate of leguminous N in the intercropping system. Our data suggest that excessive accumulation of leguminous biomass in SSA maize cropping enhances the risk for elevated N2O emissions.
We expected N2O emissions to respond more strongly to intercropping in the second year (2016), as legume mulches were applied according to their plot-wise aboveground yields in the previous year. Indeed, N2O emission rates were clearly higher in intercropping treatments on the first sampling date in 2016 (Fig. 1f, g), indicating increased N cycling in mulched plots (Campiglia et al., 2011). This difference vanished quickly, however, suggesting that the effect of intercrop mulches, even at high amounts (Table 1), on N2O emissions in the subsequent year was negligible. It is noteworthy that our estimates of the fraction of N carried over between the years were based on literature data (Table 1) and that a considerable part of the mulched N may have been lost during abundant rainfall (300 mm) early in the 2016 season before crops were sown.
Cumulative N2O emissions from intercrops, with the mineral fertilization rate halved, were comparable to those in the fully fertilized maize mono-crop in 2016. This may be partly due to the 50 % reduction in mineral N application to intercrop treatments, as found by others (Tang et al., 2017). Another reason may be that a considerable proportion of the cumulative emissions in 2016 occurred before or shortly after 3-week intercrops were sown and was thus unaffected by growing legumes. Overall, cumulative N2O emissions in 2016 were equal to or higher than those in 2015, despite reduced mineral N addition to intercrops and lower legume biomass. Ultimately, the lack of a clear emission response to legume intercropping in the second year calls for studies tracing cumulative mulching effects over multiple years and exploring their driving factors in more detail. In our study, the amount and timing of rainfall appeared to be more important for N2O emissions in the second year than the amount and carryover of legume N.
Given our finding that N2O intensity responded positively to legume biomass and its N content in a drought year with poor maize growth, intercrop species as well as sowing and harvest dates (relative to the main crop) emerge as viable management factors for controlling the accumulation of legume biomass between the maize rows and hence the risk for increased N2O emission. Legume species and cultivar in intercropping systems are known to be critical for N loss both during the intercropping and the subsequent seasons (Pappa et al., 2011; Weiler et al., 2018). The stimulating effect of crop residues on N2O emissions has been reported to depend on residue quality and soil moisture, with denitrification being the likely process (Li et al., 2016). Our study provides evidence that vigorous growth of high-yielding legume intercrops can enhance N2O emissions in years unfavorable for maize growth, whereas in years with sufficient water availability early in the growing season, maize growth is favored, preventing the excessive growth of the intercrop. Our study therefore points to optimizing the sowing date in response to the expected emergence and growth of maize as a promising option to control the growth of the intercrop and hence to deal with the risk of increased N2O emissions.
4.2 Seasonal N2O and CH4 emissions, EF, and total GHG emissions
Growing-season N2O emissions in fertilized treatments varied from 0.17 to 0.33 kg N2O-N ha−1 (2015) and 0.23 to 0.3 kg N2O-N ha−1 (2016), covering a period of 107 d (2015) and 123 d (2016) (Fig. 2) and a range of estimated total N inputs from 36.4 to 97.8 kg N ha−1 (Table 1). There are no N2O emission studies for maize–legume intercropping in the Ethiopian rift valley so far. Hickman et al. (2014a) reported N2O emissions of 0.62 and 0.81 kg N ha−1 over 99 d for 100 and 200 kg N of input per hectare, respectively, for a maize field without intercropping in humid western Kenya, which seems to be higher than the seasonal emissions we found. Baggs et al. (2006), working in the same region with maize intercropped with legumes in an agroforestry system, reported N2O emissions ranging from 0.2 to 0.6 kg N ha−1 with higher emissions in tilled intercropping treatments; our values are at the lower end of the range they reported. The largest seasonal N2O emissions for intercropping reported so far from SSA are 4.1 kg N ha−1 (84 d) after incorporating 7.4 t ha−1 of a Sesbania macroptilium mixture in humid western Kenya (Millar et al., 2004). Compared to the N2O emissions reported for humid tropical maize production systems, our data suggest that maize–legume intercropping based on mulching in the subhumid to semiarid rift valley appears to be a minor N2O source, mainly because of the relatively small amount of legume biomass mulched (Table 1). Growing-season N2O emission factors (EF) in our study ranged from 0.02 % to 0.25 % in 2015 and 0.11 % to 0.20 % in 2016 of the estimated total N input, including assumed N inputs from legume mulch as well as belowground additions and carryover between the years (Table 1). Even if the estimated EF is doubled to account for off-season emissions, it is still lower than the annual IPCC default value of 1 % N2O-N per unit of added N (IPCC, 2014). Our estimated EF values thus seem to be at the lower end of those reported by Kim et al. (2016) for SSA smallholder agriculture estimated from literature data (0.01 % to 4.1 %). The reasons for the low EF values in our study are probably the high background emissions in the fertile soil of the Hawassa University Research Farm, which supports high maize yields even in the unfertilized control (Table 1), and the low levels of N input. The soil has been used over decades for agronomic trials with various fertilization rates with and without crop residue retention and legume intercropping (e.g., Raji et al., 2019). Thus, our field trial has to be considered representative for intensive management as opposed to smallholder systems with minimal or no fertilization history.
Methane uptake by the soil in both seasons varied between 1.0 and 1.5 kg CH4-C ha−1 without showing any significant treatment effect, even though maize–legume intercrops tended to take up less CH4 than maize mono-crops (Fig. S1). The observed trend might relate to competitive inhibition of CH4 oxidation by higher availability (Le Mer and Roger, 2001; Dunfield and Knowles, 1995) in the presence of legume intercrops, even though estimated total N inputs remained below 100 kg N ha−1, which is considered a threshold for inhibition (Aronson and Helliker, 2010). Alternatively, densely growing legumes may have lowered CH4 uptake by impeding CH4 and/or O2 diffusion into the soil (Ball et al., 1997). We did not observe the stimulation of CH4 uptake by legume intercropping, which we attribute to the absence of N and P deficiency in this fertile soil. Methane uptake rates varied from 20 to 140 µg CH4-C m−2 h−1, which is in the range of rates reported previously for SSA upland soils (Pelster et al., 2017). Seasonal CH4 uptake in our experiment offset between 22 % and 69 % of the CO2 equivalents associated with N2O emissions without revealing any significant treatment effect (Fig. S2a, b), but the offset was relatively largest in the unfertilized maize mono-crop and smallest in lablab intercropping. Hence, CH4 uptake is an important component of the non-CO2 climate footprint of SSA crop production.
4.3 Legume intercropping and climate-smart agriculture
Legumes are an important N source in smallholder farming systems, where mineral fertilizers are unaffordable or unavailable. Legume intercrops maximize resource use efficiency as total productivity is often higher than in mono-cropping systems (Banik et al., 2006). Moreover, N fixed biologically by legume intercrops can partly replace synthetic N fertilizers if the release is synchronized with the nutrient demand of the cereal crop. On the other hand, surplus N from legumes may result in N losses as , NH3 and NO, N2O, or N2. Mulching and the incorporation of legume biomass has been found to increase N2O emissions under temperate conditions (Baggs et al., 2000, 2003) and under humid tropical conditions (Millar et al., 2004). Also under semiarid Mediterranean conditions, vetch (V. villosa) used as a winter catch crop and mulched in spring significantly increased N2O emissions during the fallow period, while rape did not (Sanz-Cobena et al., 2014). This was later confirmed by a 15N study, highlighting the role of N mineralization from legumes as a source of N2O (Guardia et al., 2016). None of the studies found an overall N2O-saving effect of catch crops when scaling up to the entire crop cycle, even though the latter study used reduced mineral N fertilization rates in treatments with catch crops. By contrast, reduced leaching and N2O emissions have been reported from maize intercropped with legumes in the semiarid North China Plain, which the authors attributed to enhanced N uptake by both the intercrop and main crop as well as reduced soil moisture in treatments with intercrops during the rainy season (Huang et al., 2019). This shows that legume intercrops have the potential to either increase or reduce N2O emissions with consequences for the non-CO2 footprint of cereal production and hence for the viability of intercropping as a central component of CSA (Thierfelder et al., 2017).
The legume intercrops used in our study had low C : N ratios (Table S1 in the Supplement) and can be expected to release a significant part of their N through the decomposition of roots and nodules or root exudation as well as during the decomposition of mulches (Fustec et al., 2010). The effect of mulching on N2O emissions depends on the C : N ratio, with increased emissions for residues with a low C : N ratio (Baggs et al., 2000; Shan and Yan, 2013). In line with this, N2O emissions in the intercrop treatments of our study exceeded those in the fertilized maize mono-crop on several sampling dates, both during the active growth of legumes and after mulching. Another important aspect is the amount of legume N carried over between years, which depends, among other factors, on the amount and quality of the legume and the weather between the growing seasons. Abera et al. (2014) showed that surface-placed residues of haricot bean and pigeon pea decompose quickly despite relatively dry conditions during the off-season. Vigorous rainfall at the beginning of the growing season like in 2016 (Fig. 1) could lead to dissolved N losses, which could lead to indirect N2O emissions elsewhere; this should be taken into account when evaluating intercropping as a CSA strategy.
While legume intercrops have the potential to improve cereal yields and diversify produce for smallholders in the central Ethiopian rift valley, a risk of enhanced N2O emissions remains, which became apparent as the increased “N2O intensity” of the main crop in a drought year (2015). At the same time, our study points at possibilities to counteract this trend by actively controlling legume biomass development and hence potential N input through “climate-smart” choices of legume species, sowing date and mulch amounts in response to prevailing environmental conditions. This approach, however, is complicated by annual variability in growth conditions and requires active planning for sowing and mulching time by the farmer. Our study was conducted on a relatively nutrient-rich soil (compared to typical smallholder farms), which supports high yields of both maize and leguminous intercrops. Under these conditions, intercropped legumes can potentially replace a considerable part of synthetic fertilizer, thus supporting common CSA goals. However, more studies are needed to fully explore intercropping options in the framework of CSA in the rift valley, particularly in nutrient-poor smallholder fields. Future studies on CSA approaches in the rift valley should address, in addition to greenhouse gas emissions, N runoff and soil organic matter build-up, ideally in long-term field trials with and without legume intercropping. Future studies should also attempt to combine flux measurements with inorganic N dynamics and measurements of biological N fixation. Given that seasonal N2O emission factors and intensities in our study were in the lower range of published values for SSA, intercropping appears to be a promising approach to sustainable intensification in the Ethiopian Great Rift Valley.
Flux and yield data can be accessed together with metadata through the NMBU archive at: https://doi.org/10.18710/I6BD3R (Dörsch, 2020).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-17-345-2020-supplement.
SGR and PD designed and SGR carried out the study. Both authors processed the data and wrote the paper.
The authors declare that they have no conflict of interest.
The study is part of the NORHED program “Research and capacity building in climate-smart agriculture in the Horn of Africa”. We are grateful to Teshome Geletu, Teketel Chiro and Tigist Yimer for assistance during setting up and managing the field experiment, sample collection, and preparation, as well as to Trygve Fredriksen for assistance during the analysis of samples in the laboratory at NMBU.
This research has been supported by the Norwegian Agency for Development Cooperation (Norad) under the NORHEAD program (grant number ETH-13/0016).
This paper was edited by Edzo Veldkamp and reviewed by two anonymous referees.
Abera, G., Wolde-Meskel, E., and Bakken, L. R.: Unexpected high decomposition of legume residues in dry season soils from tropical coffee plantations and crop lands, Agron. Sustain. Dev., 34, 667–676, 2014.
Almaraz, J. J., Zhou, X., Mabood, F., Madramootoo, C., Rochette, P., Ma, B.-L., and Smith, D. L.: Greenhouse gas fluxes associated with soybean production under two tillage systems in southwestern Quebec, Soil Till. Res., 104, 134–139, 2009.
Aronson, E. L. and Helliker, B. R.: Methane flux in non-wetland soils in response to nitrogen addition: a meta-analysis, Ecology, 91, 3242–3251, 2010.
Arslan, A., Mccarthy, N., Lipper, L., Asfaw, S., Cattaneo, A., and Kokwe, M.: Climate Smart Agriculture? Assessing the Adaptation Implications in Zambia, J. Agr. Econ., 66, 753–780, 2015.
Baggs, E. M., Chebii, J., and Ndufa, J. K.: A short-term investigation of trace gas emissions following tillage and no-tillage of agroforestry residues in western Kenya, Soil Till. Res., 90, 69–76, 2006.
Baggs, E. M., Rees, R. M., Smith, K. A., and Vinten, A. J. A.: Nitrous oxide emission from soils after incorporating crop residues, Soil Use Manage., 16, 82–87, 2000.
Baggs, E. M., Stevenson, M., Pihlatie, M., Regar, A., Cook, H., and Cadisch, G. J.: Nitrous oxide emissions following application of residues and fertiliser under zero and conventional tillage, Plant Soil, 254, 361–370, 2003.
Bakken, L. R., Bergaust, L., Liu, B., and Frostegård, Å.: Regulation of denitrification at the cellular level: a clue to the understanding of N2O emissions from soils, Philos T. R. Soc. Lon. B, 367, 1226–1234, https://doi.org/10.1098/rstb.2011.0321, 2012.
Ball, B. C., Smith, K. A., Klemedtsson, L., Brumme, R., Sitaula, B. K., Hansen, S., Prieme, A., Macdonald, J., and Horgan, G. W.: The influence of soil gas transport properties on methane oxidation in a selection of northern European soils, J. Geophys. Res., 102, 23309–23317, 1997.
Banik, P., Midya, A., Sarkar, B. K., and Ghose, S. S.: Wheat and chickpea intercropping systems in an additive series experiment: Advantages and weed smothering, Eur. J. Agron., 24, 325–332, 2006.
Bédard, C. and Knowles, R.: Physiology, biochemistry, and specific inhibitors of CH4, , and CO oxidation by methanotrophs and nitrifiers, Microbiol. Rev., 53, 68–84, 1989.
Bedoussac, L., Journet, E.-P., Hauggaard-Nielsen, H., Naudin, C., Corre-Hellou, G., Jensen, E. S., Prieur, L., and Justes, E. J. A. F. S. D.: Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review, Agron. Sustain. Dev., 35, 911–935, 2015.
Blanc E. and Strobl, E.: The impact of climate change on cropland productivity: evidence from satellite based products at the river basin scale in Africa, Climatic Change, 117, 873–890, 2013.
Campiglia, E., Mancinelli, R., Radicetti, E., and Marinari, S.: Legume cover crops and mulches: effects on nitrate leaching and nitrogen input in a pepper crop (Capsicum annuum L.), Nutr. Cycl. Agroecosys., 89, 399–412, 2011.
Carranca, C., Torres, M. O., and Madeira, M.: Underestimated role of legume roots for soil N fertility, Agro. Sustain. Dev., 35, 1095–1102, 2015.
Carruthers, K., Prithiviraj, B., Fe, Q., Cloutier, D., Martin, R. C., and Smith, D. L.: Intercropping corn with soybean, lupin and forages: yield component responses, Eur. J. Agron., 12, 103–115, 2000.
Davidson, E. A., Keller, M., Erickson, H. E., Verchot, L. V., and Veldkamp, E.: Testing a Conceptual Model of Soil Emissions of Nitrous and Nitric Oxides: Using two functions based on soil nitrogen availability and soil water content, the hole-in-the-pipe model characterizes a large fraction of the observed variation of nitric oxide and nitrous oxide emissions from soils, BioScience, 50, 667–680, 2000.
de Jager, I., Borgonjen-van den Berg, K. J., Giller, K. E., and Brouwer, I. D.: Current and potential role of grain legumes on protein and micronutrient adequacy of the diet of rural Ghanaian infants and young children: using linear programming, Nutr. J., 18, 12, https://doi.org/10.1186/s12937-019-0435-5, 2019.
Dick, J., Kaya, B., Soutoura, M., Skiba, U., Smith, R., Niang, A., and Tabo, R.: The contribution of agricultural practices to nitrous oxide emissions in semi-arid Mali, Soil Use Manage., 24, 292–301, 2008.
Dörsch, P.: Replication Data for: Effect of legume intercropping on N2O emissions and CH4 uptake during maize production in the Great Rift Valley, Ethiopia, https://doi.org/10.18710/I6BD3R, DataverseNO, V1, 2020.
Drury, C. F., Mckenney, D. J., and Findlay, W. I.: Relationships between denitrification, microbial biomass and indigenous soil properties, Soil Biol. Biochem., 23, 751–755, 1991.
Dunfield, P. and Knowles, R.: Kinetics of inhibition of methane oxidation by nitrate, nitrite, and ammonium in a humisol, Appl. Environ. Microb., 61, 3129, 1995.
Ehrmann, J. and Ritz, K.: Plant soil interactions in temperate multi-cropping production systems, Plant Soil, 376, 1–29, 2014.
Fageria, N. K., Moreira, A., Moraes, L. A. C., and Moraes, M. F.: Root growth, nutrient uptake, and nutrient-use efficiency by roots of tropical legume cover crops as influenced by phosphorus fertilization, Commun. Soil Sci. Plan., 45, 555–569, 2014.
Flessa, H., Dörsch, P., and Besse, F.: Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in Southern Germany, J. Geophys. Res., 100, 23115–23124, 1995.
Fustec, J., Lesuffleur, F., Mahieu, S., and Cliquet, J.-B. J. A. F. S. D.: Nitrogen rhizodeposition of legumes. A review, Agron. Sustain. Dev., 30, 57–66, 2010.
Guardia, G., Abalos, D., García-Marco, S., Quemada, M., Alonso-Ayuso, M., Cárdenas, L. M., Dixon, E. R., and Vallejo, A.: Effect of cover crops on greenhouse gas emissions in an irrigated field under integrated soil fertility management, Biogeosciences, 13, 5245–5257, https://doi.org/10.5194/bg-13-5245-2016, 2016.
Harrison-Kirk, T., Beare, M. H., Meenken, E. D., and Condron, L. M.: Soil organic matter and texture affect responses to dry/wet cycles: Effects on carbon dioxide and nitrous oxide emissions, Soil Biol. Biochem., 57, 43–55, 2013.
Hickman, J. E., Palm, C. A., Mutuo, P., Melillo, J. M., and Tang, J.: Nitrous oxide (N2O) emissions in response to increasing fertilizer addition in maize (Zea mays L.) agriculture in western Kenya, Nutr. Cycl. Agroecosys., 100, 177–187, 2014a.
Hickman, J. E., Scholes, R. J., Rosenstock, T. S., Perez Garcia-Pando, C., and Nyamangara, J.: Assessing non-CO2 climate-forcing emissions and mitigation in sub-Saharan Africa, Curr. Opin. Env. Sust., 9–10, 65–72, 2014b.
Ho, A., Reim, A., Kim, S. Y., Meima-Franke, M., Termorshuizen, A., De Boer, W., Van der Putten, W. H., and Bodelier, P. L. E.: Unexpected stimulation of soil methane uptake as emergent property of agricultural soils following bio-based residue application, Glob. Change Biol., 21, 3864–3879, 2015.
Huang, J., Sui, P., Gao, W., and Chen, Y.: Effect of maize-soybean intercropping on soil nitrous oxide emissions in silt loam soil of the North China Plain, Pedosphere, 29, 764–772, 2019.
IPCC: Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., Kriemann, B., Savolainen, J., Schlömer, S., von Stechow, C., Zwickel, T., and Minx, J. C., Cambridge University Press, Cambridge, UK and New York, NY, USA, 2014.
Jensen, E. S. and Hauggaard-Nielsen, H.: How can increased use of biological N2 fixation in agriculture benefit the environment?, Plant Soil, 252, 177–186, 2003.
Khalil, K., Mary, B., and Renault, P.: Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration, Soil Biol. Biochem., 36, 687–699, 2004.
Kim, D.-G., Thomas, A. D., Pelster, D., Rosenstock, T. S., and Sanz-Cobena, A.: Greenhouse gas emissions from natural ecosystems and agricultural lands in sub-Saharan Africa: synthesis of available data and suggestions for further research, Biogeosciences, 13, 4789–4809, https://doi.org/10.5194/bg-13-4789-2016, 2016.
Laanbroek, H. J. and Bodelier, P. L. E.: Nitrogen as a regulatory factor of methane oxidation in soils and sediments, FEMS Microbiol. Ecol., 47, 265–277, 2004.
Le Mer, J. and Roger, P.: Production, oxidation, emission and consumption of methane by soils: A review, Eur. J Soil Biol., 37, 25–50, 2001.
Li, X., Sørensen, P., Olesen, J. E., and Petersen, S. O.: Evidence for denitrification as main source of N2O emission from residue-amended soil, Soil Biol. Biochem., 92, 153–160, 2016.
Makate, C., Makate, M., Mutenje, M., Mango, N., and Siziba, S.: Synergistic impacts of agricultural credit and extension on adoption of climate-smart agricultural technologies in southern Africa, Environmental Development, 32, 100458, https://doi.org/10.1016/j.envdev.2019.100458, 2019.
Millar, N., Ndufa, J. K., Cadisch, G., and Baggs, E. M.: Nitrous oxide emissions following incorporation of improved-fallow residues in the humid tropics, Global Biogeochem. Cy., 18, GB1032, https://doi.org/10.1029/2003GB002114, 2004.
Nadeem, S., Bakken, L. R., Frostegård, Å., Gaby, J. C., and Dörsch, P.: Liming enhances nitrification and increases its N2O yield but reduces soil N2O emissions from coupled nitrification-denitrification through increased N2O reductase activity, Soil Biol. Biochem., in review, 2019.
Neufeldt, H., Jahn, M., Campbell, B. M., Beddington, J. R., Declerck, F., De Pinto, A., Gulledge, J., Hellin, J., Herrero, M., Jarvis, A., Lezaks, D., Meinke, H., Rosenstock, T., Scholes, M., Scholes, R., Vermeulen, S., Wollenberg, E., and Zougmore, R.: Beyond climate-smart agriculture: toward safe operating spaces for global food systems, Agriculture & Food Security, 2, 12, https://doi.org/10.1186/2048-7010-2-12, 2013.
Odhiambo, J. J. O.: Decomposition and nitrogen release by green manure legume residues in different soil types, Afr. J. Agr. Res., 5, 90–96, 2010.
Ojiem, J. O., Vanlauwe, B., De Ridder, N., and Giller, K. E.: Niche-based assessment of contributions of legumes to the nitrogen economy of Western Kenya smallholder farms, Plant Soil, 292, 119–135, 2007.
Pappa, V. A., Rees, R. M., Walker, R. L., Baddeley, J. A., and Watson, C. A.: Nitrous oxide emissions and nitrate leaching in an arable rotation resulting from the presence of an intercrop, Agr. Ecosyst. Environ., 141, 153–161, 2011.
Pelster, D., Rufino, M., Rosenstock, T., Mango, J., Saiz, G., Diaz-Pines, E., Baldi, G., and Butterbach-Bahl, K.: Smallholder farms in eastern African tropical highlands have low soil greenhouse gas fluxes, Biogeosciences, 14, 187–202, https://doi.org/10.5194/bg-14-187-2017, 2017.
Raji, S. G., Tzanakakis, V., and Dörsch, P.: Bradyrhizobial inoculation and P application effects on haricot and mung beans in the Ethiopian Rift Valley, Plant Soil, 442, 271–284, https://doi.org/10.1007/s11104-019-04170-2, 2019.
Rochette, P. and Eriksen-Hamel, N. S.: Chamber measurements of soil nitrous oxide flux: Are absolute values reliable?, Soil Sci. Soc. Am. J., 72, 331–342, 2008.
Rochette, P. and Janzen, H. H.: Towards a revised coefficient for estimating N2O emissions from legumes, Nutr. Cycl. Agroecosys., 73, 171–179, 2005.
Russenes, A. L., Korsaeth, A., Bakken, L. R., and Dörsch, P.: Spatial variation in soil pH controls off-season N2O emission in an agricultural soil, Soil Biol. Biochem., 99, 36–46, 2016.
Sant'anna, S. A. C., Martins, M. R., Goulart, J. M., Araujo, S. N., Araujo, E. S., Zaman, M., Jantalia, C. P., Alves, B. J. R., Boddey, R. M., and Urquiaga, S.: Biological nitrogen fixation and soil N2O emissions from legume residues in an Acrisol in SE Brazil, Geoderma Regional, 15, e00196, https://doi.org/10.1016/j.geodrs.2018.e00196, 2018.
Sanz-Cobena, A., Garcia-Marco, S., Quemada, M., Gabriel, J. L., Almendros, P., and Vallejo, A.: Do cover crops enhance N2O, CO2 or CH4 emissions from soil in Mediterranean arable systems?, Sci. Total Environ., 466–467, 164–174, 2014.
Schaufler, G., Kitzler, B., Schindlbacher, A., Skiba, U., Sutton, M. A., Zechmeister-Boltenstern, S.: Greenhouse gas emissions from European soils under different land use: effects of soil moisture and temperature, Eur. J. Soil Sci., 61, 683–696, 2010.
Schlüter, S., Zawallich, J., Vogel, H.-J., and Dörsch, P.: Physical constraints for respiration in microbial hotspots in soil and their importance for denitrification, Biogeosciences, 16, 3665–3678, https://doi.org/10.5194/bg-16-3665-2019, 2019.
Schwenke, G. D., Herridge, D. F., Scheer, C., Rowlings, D. W., Haigh, B. M., and Mcmullen, K. G.: Greenhouse gas (N2O and CH4) fluxes under nitrogen-fertilised dryland wheat and barley on subtropical Vertosols: Risk, rainfall and alternatives, Soil Res., 54, 634–650, 2016.
Shan, J. and Yan, X.: Effects of crop residue returning on nitrous oxide emissions in agricultural soils, Atmos. Environ., 71, 170–175, 2013.
Sime, G. and Aune, J. B.: Sustainability of Improved Crop Varieties and Agricultural Practices: A Case Study in the Central Rift Valley of Ethiopia, Agriculture, 8, 1–16, https://doi.org/10.3390/agriculture8110177, 2018.
Tang, Y. L., Yu, L. L., Gaun, A. M., Zhou, X. Y., Wang, Z. G., Gou, Y. G., and Wang, J. W.: Soil mineral nitrogen and yield-scaled soil N2O emissions lowered by reducing nitrogen application and intercropping with soybean for sweet maize production in southern China, J. Integr. Agr., 16, 2586–2596, 2017.
Thierfelder, C., Chivenge, P., Mupangwa, W., Rosenstock, T. S., Lamanna, C., and Eyre, J. X. J. F. S.: How climate-smart is conservation agriculture (CA)? – its potential to deliver on adaptation, mitigation and productivity on smallholder farms in southern Africa, Food Secur., 9, 537–560, 2017.
Wanyama, I., Pelster, D. E., Butterbach-Bahl, K., Verchot, L. V., Martius, C., and Rufino, M. C.: Soil carbon dioxide and methane fluxes from forests and other land use types in an African tropical montane region, Biogeochemistry, 143, 171–190, 2019.
Weiler, D. A., Giacomini, S. J., Recous, S., Bastos, L. M., Pilecco, G. E., Dietrich, G., and Aita, C.: Trade-off between C and N recycling and N2O emissions of soils with summer cover crops in subtropical agrosystems, Plant Soil, 433, 213–225, 2018.
Wrage-Mönnig, N., Horn, M. A., Well, R., Müller, C., Velthof, G., and Oenema, O.: The role of nitrifier denitrification in the production of nitrous oxide revisited, Soil Biol. Biochem., 123, A3–A16, 2018.
Wu, D., Wei, Z., Well, R., Shan, J., Yan, X., Bol, R., and Senbayram, M.: Straw amendment with nitrate-N decreased ratio but increased soil N2O emission: A case study of direct soil-born N2 measurements, Soil Biol. Biochem., 127, 301–304, 2018.