the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Coccolithophore biodiversity controls carbonate export in the Southern Ocean
Andrés S. Rigual Hernández
Thomas W. Trull
Scott D. Nodder
José A. Flores
Helen Bostock
Fátima Abrantes
Ruth S. Eriksen
Francisco J. Sierro
Diana M. Davies
Anne-Marie Ballegeer
Miguel A. Fuertes
Lisa C. Northcote
Southern Ocean waters are projected to undergo profound changes in their physical and chemical properties in the coming decades. Coccolithophore blooms in the Southern Ocean are thought to account for a major fraction of the global marine calcium carbonate (CaCO3) production and export to the deep sea. Therefore, changes in the composition and abundance of Southern Ocean coccolithophore populations are likely to alter the marine carbon cycle, with feedbacks to the rate of global climate change. However, the contribution of coccolithophores to CaCO3 export in the Southern Ocean is uncertain, particularly in the circumpolar subantarctic zone that represents about half of the areal extent of the Southern Ocean and where coccolithophores are most abundant. Here, we present measurements of annual CaCO3 flux and quantitatively partition them amongst coccolithophore species and heterotrophic calcifiers at two sites representative of a large portion of the subantarctic zone. We find that coccolithophores account for a major fraction of the annual CaCO3 export, with the highest contributions in waters with low algal biomass accumulations. Notably, our analysis reveals that although Emiliania huxleyi is an important vector for CaCO3 export to the deep sea, less abundant but larger species account for most of the annual coccolithophore CaCO3 flux. This observation contrasts with the generally accepted notion that high particulate inorganic carbon accumulations during the austral summer in the subantarctic Southern Ocean are mainly caused by E. huxleyi blooms. It appears likely that the climate-induced migration of oceanic fronts will initially result in the poleward expansion of large coccolithophore species increasing CaCO3 production. However, subantarctic coccolithophore populations will eventually diminish as acidification overwhelms those changes. Overall, our analysis emphasizes the need for species-centred studies to improve our ability to project future changes in phytoplankton communities and their influence on marine biogeochemical cycles.
- Article
(2460 KB) - Full-text XML
-
Supplement
(291 KB) - BibTeX
- EndNote
The emissions of carbon dioxide (CO2) into the atmosphere by anthropogenic industrial activities over the past 200 years are inducing a wide range of alterations in the marine environment (Pachauri et al., 2014). These include ocean warming, shallowing of mixed layer depths, changes in nutrient supply to the photic zone and decreasing carbonate ion concentrations and pH of the surface ocean, a process known as ocean acidification (Rost and Riebesell, 2004; IPCC, 2013). Substantial evidence from CO2 manipulation experiments indicates that many species of corals, pteropods, planktonic foraminifera and coccolithophores will reduce their calcification rates under future ocean acidification scenarios (Bijma et al., 2002; Langdon and Atkinson, 2005 among others; Orr et al., 2005; Bach et al., 2015; Meyer and Riebesell, 2015). Owing to their moderate alkalinity and cold temperatures, Southern Ocean waters are projected to become undersaturated with respect to aragonite no later than 2040 and to calcite by the end of the century (Cao and Caldeira, 2008; McNeil and Matear, 2008; Shadwick et al., 2013). This decline in the saturation state of carbonate, together with other changes in carbonate chemistry speciation, will enhance dissolution of both aragonite and calcite shells and will make the biological precipitation of carbonate difficult in some marine calcifying organisms (Fabry et al., 2008; Gattuso and Hansson, 2011). Since such thresholds will be reached sooner in polar regions, Southern Ocean ecosystems have been proposed as bellwethers for prospective impacts of ocean acidification on marine organisms at mid- and low latitudes (Fabry et al., 2009).
Coccolithophores are a major component of phytoplankton communities in the Southern Ocean, particularly in its northernmost province, the subantarctic zone, where they often exhibit maximum abundances and diversity (e.g. Gravalosa et al., 2008; Saavedra-Pellitero et al., 2014; Malinverno et al., 2015; Charalampopoulou et al., 2016). Coccolithophores play an important and complex role in the Southern Ocean carbon cycle (Salter et al., 2014). On the one hand, the production of calcite platelets (termed coccoliths) decreases the alkalinity of surface waters, thereby reducing the atmospheric uptake of CO2 from the atmosphere into the surface ocean. On the other hand, the production of organic matter through photosynthesis, and its subsequent transport to depth in settling particles, enhances carbon sequestration via the biological carbon pump (Volk and Hoffert, 1985). Additionally, due to their high density and slow dissolution, coccoliths act as an effective ballast for organic matter, increasing organic carbon sequestration depths (Buitenhuis et al., 2001; Boyd and Trull, 2007; Ziveri et al., 2007). Therefore, changes in the abundance, composition and distribution of coccolithophores could have an extensive impact on ocean nutrient stoichiometry, carbon sequestration and nutrition for higher trophic levels in the Southern Ocean (Deppeler and Davidson, 2017).
The remoteness and vastness of the Southern Ocean, together with the inherent temporal and spatial variability of pelagic ecosystems, hampers accurate characterization and quantification of Southern Ocean phytoplankton communities. Advances in satellite technology and modelling algorithms have allowed a circumpolar and year-round coverage of the seasonal evolution of major phytoplankton functional groups within the Southern Ocean (e.g. Alvain et al., 2013; Hopkins et al., 2015; Rousseaux and Gregg, 2015). In particular, ocean-colour satellite reflectance observations have been used to quantitatively estimate coccolithophore particulate inorganic carbon (PIC) concentrations throughout the Southern Ocean (Gordon et al., 2001; Balch et al., 2005b). These satellite estimates suggest apparent high PIC values during summer near the major Southern Ocean fronts attributed to coccolithophores (Balch et al., 2011, 2016). This band of elevated reflectance and PIC that encircles the entire Southern Ocean was termed the “Great Calcite Belt” by these authors. However, comparison of satellite remote-sensing data with ship-based observations (Holligan et al., 2010; Trull et al., 2018) indicates that satellite ocean-colour-based PIC estimates could be unreliable, particularly in Antarctic waters where they erroneously suggest high PIC abundances. Shipboard observations, on the other hand, provide a detailed picture of phytoplankton community composition and structure but are dispersed, both temporally and geographically, and provide rather heterogenous data in terms of taxonomic groups investigated and the sampling scales and methodologies used (e.g. Kopczynska et al., 2001; de Salas et al., 2011; Poulton et al., 2013; Patil et al., 2017, among others). In situ year-round monitoring of key strategic regions is critically needed to establish baselines of phytoplankton community composition and abundance and to validate and improve ocean biogeochemical models (Rintoul et al., 2012). This information is also essential if we are to detect possible climate-driven changes in the structure of phytoplankton communities that could influence the efficiency of the biological carbon pump, with consequent feedbacks to the rate of deep-water carbon sequestration and global climate change (Le Quéré et al., 2007; Deppeler and Davidson, 2017).
Here, we document coccolithophore and carbonate particle fluxes collected over a year by four sediment trap records deployed at two strategic locations of the Australian and New Zealand sectors of the Southern Ocean considered representative of a large portion of the SAZ (see Sect. 2.2 for further details). Our measurements provide coccolith mass estimates of the main coccolithophore species and quantitatively partition annual carbonate fluxes amongst coccolithophore species and heterotrophic calcifiers. We find that coccolithophores are a major vector for CaCO3 export out of the mixed layer and that the largest contribution to CaCO3 export is not from the most abundant species Emiliania huxleyi but rather from larger coccolithophores species with substantially different physiological traits (e.g. Calcidiscus leptoporus). Our results emphasize the urgent need for diagnostic fitness response experiments on other coccolithophore species aside from E. huxleyi (e.g. Feng et al., 2017) in order to be able to predict the impacts of anthropogenically induced changes in Southern Ocean ecosystems and biological carbon uptake mechanisms.
2.1 Oceanographic setting
The SAZ alone accounts for more than half of the Southern Ocean area (Orsi et al., 1995) and represents a transitional boundary between the warm, oligotrophic waters of the subtropical gyres to the north and the cold, silicate-rich waters south of the polar front (PF). The SAZ is arguably the largest high-nutrient, low-chlorophyll (HNLC) province in the world's ocean and is central to the linkages between the ocean–atmosphere CO2 exchange and climate. The deep winter convection in the SAZ, which exceeds 400 m, results in the formation of high-oxygen water masses known as Subantarctic Mode Water and Antarctic Intermediate Water that connect the upper and lower limbs of the global overturning circulation (Sloyan and Rintoul, 2001a, b). The formation of these water masses is responsible for the sequestration of a large fraction of anthropogenic CO2 (Sabine et al., 2004), with an estimated 1 Gt C yr−1 transported to intermediate depths annually (Metzl et al., 1999). Macronutrient concentrations display pronounced seasonal changes in the SAZ, with fully replete levels during winter to substantial depletion during summer, particularly for silicate (Dugdale et al., 1995; Rintoul and Trull, 2001; Bowie et al., 2011). The phytoplankton community in the subantarctic zone is dominated by pico- and nannoplankton including cyanobacteria, coccolithophores and autotrophic flagellates, with lower abundances of diatoms than polar waters south the polar front (Chang and Gall, 1998; Kopczynska et al., 2001; de Salas et al., 2011; Rigual-Hernández et al., 2015b; Eriksen et al., 2018).
2.2 Field experiments
Here we report on the coccolithophore and biogeochemical fluxes collected over a year at the Australian Southern Ocean Time Series (SOTS) observatory (Trull et al., 2010) and the New Zealand Subantarctic Mooring (SAM) site (Nodder et al., 2016) (Fig. 1). The SOTS observatory is located in the abyssal plane of the central SAZ approximately 530 km southwest of Tasmania (46∘56′ S, 142∘15′ E) within an anticyclonic gyre in a region characterized by weak circulation (Trull et al., 2001; Herraiz-Borreguero and Rintoul, 2011). The SOTS site was equipped with three vertically moored, conical time-series sediment traps (McLane Parflux Mk7G-21) placed at ∼1000, 2000 and 3800 m depth between August 2011 and July 2012. The physical, chemical and biological parameters of the SOTS site are regarded as representative for large portion of the Indian and Australian sectors of the SAZ (∼90 and 140∘ E; Trull et al., 2001). The SAM site is located in the Bounty Trough in the subantarctic waters south-east of New Zealand (46∘40′ S, 178∘30′ E) and was equipped with a conical, time-incremental sediment trap (McLane Parflux Mk7G-21) placed at 1500 m depth, with samples used in the present study collected between November 2009 and November 2010. The SAM site is considered to be representative of a wide area of the northern sector of the SAZ off eastern New Zealand, approximately 171∘ E to 179∘ W and 45 to 47∘ S (Law et al., 2014; Fig. 1). Full details of the field experiments from these two localities in the Australian and New Zealand sectors of the SAZ can be found in Trull et al. (2001) and Nodder et al. (2016), respectively.
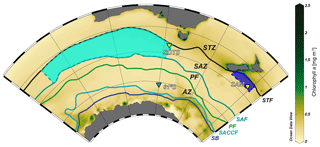
Figure 1Chlorophyll a composite map of the Australian–New Zealand sector of the Southern Ocean (July 2002 to July 2012) from the MODIS Aqua sensor showing the location of the sediment trap moorings sites: SOTS, 61∘ S and SAM. The regions for which the SOTS and SAM sites are representative are marked with light- and dark-blue areas, respectively. Abbreviations: subtropical zone – STZ, subtropical front – STF, subantarctic zone – SAZ, subantarctic front – SAF, polar frontal zone – PFZ, polar front – PF, Antarctic zone – AZ, southern Antarctic Circumpolar Current front – SACCF, and southern boundary of the ACC – SB. Oceanic fronts follow Orsi et al. (1995). Ocean Data View software (Schlitzer, 2018) was used to generate this figure.
2.3 Sample processing
In short, the recovered trap bottles were refrigerated upon recovery and then allowed to settle. The sample slurry was then wet-sieved through a 1 mm screen in the case of SOTS (no attempt to extract zooplankton “swimmers” was made for the <1 mm fraction analysed here) and through a 200 µm sieve to remove swimmers for the SAM site. The remaining fraction was then split using a McLane wet sample divider; the SOTS samples were subdivided into 1∕10 aliquots while 1 / 5 splits were made for the SAM samples. For the SOTS samples, a total of 55 samples were processed for calcareous nannoplankton analysis. The 1∕10 splits dedicated to phytoplankton analysis were further subdivided into four aliquots with the McLane splitter. One aliquot was used for calcareous nannoplankton analysis, and the remaining three were kept refrigerated for biomarker and non-calcareous microplankton analyses. In the case of the SAM samples, the one-fifth aliquots were further subdivided into five sub-splits, and one of those was used for calcareous nannoplankton analysis. Two different types of glass slides per sample were prepared. The first preparation was used for the estimation of coccosphere and calcareous dinocyst (calcispheres of thoracosphaerids) fluxes and for coccolith imaging. A volume ranging between 1000 and 5000 µL of the raw sample was mounted on a glass slide using Canada balsam following Flores and Sierro (1997). This technique produces random settling of the coccoliths for microscopic identification and enumeration. The second type of glass slide was prepared following a modified protocol for non-destructive disintegration of aggregates modified from Bairbakhish et al. (1999). The objective of this chemical treatment is to reduce biases in the coccolith flux estimations associated with the presence of different types of aggregates and coccospheres (Bairbakhish et al., 1999). In brief, a total of 2000 µL was extracted from the aliquot for calcareous nannoplankton analysis and then treated with a solution comprising 900 µL sodium carbonate and sodium hydrogen carbonate, 100 µL ammonia (25 %), and 2000 µL hydrogen peroxide (25 %). The sample was agitated for 10 s every 10 min and this process was repeated over an hour. Then, the reaction was stopped with catalase enzyme, and samples were allowed to settle for at least 48 h before preparation on microscope slides. pH controls indicate that the solution kept pH levels near 9, therefore precluding coccolith dissolution. Finally, trap samples were mounted on microscope slides following the same decantation method as used for the first type of glass slides (i.e. Flores and Sierro, 1997).
2.4 Determination of CaCO3 fluxes
A detailed description of the geochemical analytical procedures for the SOTS samples is provided in Trull et al. (2001) and Rigual-Hernández et al. (2015a), while more detailed procedures of the SAM trap can be found in Nodder et al. (2016). In short, for the SOTS site three of the 1∕10 splits were filtered onto 0.45 pore size filters. Then the material was removed from the filter as a wet cake of material, dried at 60 ∘C and ground in an agate mortar. This material was used to determine the total mass and composition of the major components of the flux. Particulate inorganic carbon content was measured by closed system acidification with phosphoric acid and coulometry. For the SAM site, a one-fifth split was analysed for elemental calcium (Ca) concentration using inductively coupled plasma mass spectrometry (ICP-MS) techniques. The samples were oven-dried, digested in nitric ∕ hydrochloric acid and then analysed according to the methods under US EPA 200.2. Ca was used to estimate CaCO3 content in the samples assuming a 1 : 1 molar ratio in CaCO3.
2.5 Quantification and characterization of coccolithophore sinking assemblages
Qualitative and quantitative analyses of coccospheres and coccoliths were performed using a Nikon Eclipse 80i polarized light microscope at 1000× magnification. The taxonomic concepts of Young et al. (2003) and the Nannotax website (Young et al., 2019) were used. A target of 100 coccospheres and 300 coccoliths was established; however, owing to the pronounced seasonality in coccolithophore export, there were some periods with very low abundance of coccospheres in the samples, and therefore the target of 100 coccospheres was not always met. Coccosphere and coccolith species counts were then transformed into relative abundances and daily fluxes using the following formula:
where F is the coccolith flux, N is the number of coccoliths, A is the area of the Petri dish, n is the number of fields of view, a is the area of a field of view, V is the dilution volume, S is the sample split, d is the number of days of collection and T is the sediment trap aperture area.
2.6 Determination of coccolith mass and size
Birefringence and morphometric methods are the two most commonly used approaches for estimating the calcite content of isolated coccoliths. The circularly polarized light-microscopy-based technique (Fuertes et al., 2014) is based on the systematic relationship between the thickness of a given calcite particle (in the thickness range of 0–1.55 mm) and the first-order polarization colours that it displays under polarized light (Beaufort, 2005; Beaufort et al., 2014; Bolton et al., 2016). The advantages of this approach are that (i) it directly measures complete coccoliths with no assumptions regarding their shape or thickness and (ii) it allows for quantification of calcite losses associated with missing parts or etching of the coccoliths. The disadvantages of this technique are the errors associated with the coccolith calcite calibration and their consequent effect on the coccolith mass estimates (Fuertes et al., 2014; González-Lemos et al., 2018). The morphometric approach, on the other hand, allows better taxonomic identification of the coccoliths and has smaller errors in the length measurements (∼0.1 to 0.2 µm; Poulton et al., 2011). However, this method does not allow for direct measurement of coccolith thickness and assumes identical shape and width proportions for all specimens of the same species, among other uncertainties (see Young and Ziveri, 2000, for a review). Since the two methods have different associated errors (Poulton et al., 2011), we applied both approaches to our coccolith flux data in order to obtain two independent estimates of the fractional contribution of coccolithophores species to total carbonate export in the SAZ.
For the birefringence-based approach, a minimum of 50 coccoliths of each of the main coccolithophore species were imaged using a Nikon Eclipse LV100 POL light microscope equipped with circular polarization and a digital camera (Nikon DS-Fi1 8 bit colour). The only exception was E. huxleyi, for which coccolith mass values had already been estimated in all the same samples at high resolution by Rigual-Hernández et al. (2020). For the minor components of the flux assemblage, a lower number of coccoliths were measured (Table 1). A photograph of the same apical rhabdolith of the genus Acanthoica was taken and used for calibration at the beginning of each imagining session during which microscopy light and camera settings were kept constant. A different number of fields of view of multiple samples representative of different seasons were photographed until the target number of coccoliths for each species was reached. Photographs were then analysed by the image-processing software C-Calcita. The output files for single coccoliths were visually selected and classified into the lowest possible taxonomic level. Length and weight measurements were automatically determined by C-Calcita software. Morphometric measurements of all the species are summarized in Table 1. For further methodological details, see Fuertes et al. (2014) and Bolton et al. (2016).
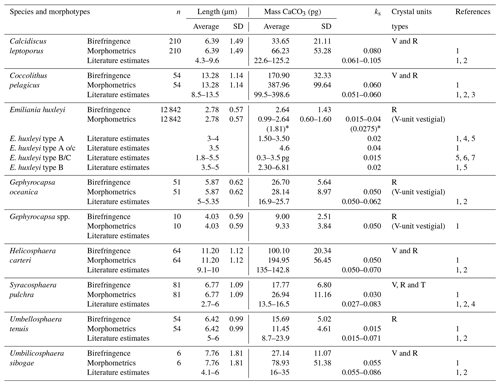
Table 1Coccolith mass estimates of the main coccolithophore species found at the SOTS and SAM sites using birefringence (C-Calcita) and morphometrics. Additionally, length and mass estimates from the literature are also listed for most species. References: (1) Young and Ziveri (2000), (2) Beaufort and Heussner (1999), (3) Samtleben and Bickert (1990), (4) Poulton et al. (2010), (5) Poulton et al. (2011), (6) Holligan et al. (2010) and (7) Charalampopoulou et al. (2016). * Coccolith mass range obtained applying the minimum and maximum ks values for E. huxleyi found in the literature (i.e. 0.015 and 0.04, respectively).
The second approach consisted of performing morphometric measurements on the coccoliths followed by the estimation of their coccolith mass assuming a systematic relation between length and thickness (Young and Ziveri, 2000). Young and Ziveri (2000) proposed that the calcite content of a given coccolith could be estimated using the following formula:
where 2.7 is the density of calcite (CaCO3; pg µm3), ks is a shape constant that varies between species and morphotypes and whose value is based on the reconstruction of coccolith cross profiles, and l is the distal shield length (DSL). In order to undertake coccolith measurements on the same coccoliths used for the birefringence-based approach, we employed the distal shield length values measured by C-Calcita using circularly polarized light instead of morphometric measurements on scanning electron micrographs (SEMs) as made in Young and Ziveri (2000).
Since coccolith distal shield length has been reported to be systematically underestimated using cross-polarized light microscopy (e.g. D'Amario et al., 2018), we evaluated the possible errors in the DSL measurements made by C-Calcita. For this assessment, we measured 40 detached coccoliths of C. leptoporus under the SEMs from samples of the SOTS sediment traps using the image-processing software ImageJ. Average DSL measurements under the SEMs were then compared with those made by C-Calcita on 40 randomly selected C. leptoporus coccoliths. The average coccolith length obtained with the SEM analysis (6.37±1.02, n=40) was ∼4 % shorter than that estimated with C-Calcita (6.62±1.47, n=40). Therefore, we assumed the error for the DSL measurements with circularly polarized light is <5 %. Given the low numbers of the rest of species in the samples, we considered that this error is applicable for the rest of the taxa measured in the current study. The subtle differences in coccolith distal length measurements between techniques are most likely due to the fact that the peripheral limit of the coccolith shield under the circularly polarized light microscope (LM) is not as sharp as is the case for SEM images. It follows that differences in DSL measurements between SEM and LM techniques will be likely similar or smaller in the case of larger species. Since the majority of coccolith species identified in the current study display a similar (e.g. Gephyrocapsa oceanica, Syracosphaera pulchra, Umbellosphaera tenuis and Umbilicosphaera sibogae) or larger size (e.g. Coccolithus pelagicus and Helicosphaera carteri) than C. leptoporus, it could be assumed that the <5 % error on DSL estimates for C. leptoporus is applicable for the rest of the species found in the current study. For the ks value of each taxa, data from the literature were employed (Table 1). E. huxleyi assemblages in the SAZ are composed of a mixture of five different morphotypes: A, A overcalcified, B, B/C and C, each of which is characterized by different shape factors (ks). Since ks is not available for all the morphotypes found in the SAZ and it is not possible to differentiate between morphotypes in our light microscopy images, we used the mean shape factor constant for E. huxleyi (i.e. ks=0.0275) to provide a range of coccolith mass estimates for this species (Table 1 and Fig. 4).
2.7 Calculation of annual estimates
Since the trap collection periods encompassed a period shorter than a calendar year, annual estimates of coccolith and CaCO3 fluxes and species relative abundances had to be estimated. For the SOTS site, a total of 336 d were sampled for the 1000 and 2000 m traps and 338 d for the 3800 m. Since the unobserved interval occurred in winter, the missing sampling period was filled using an average flux value of the winter cups (first and last trap bottles). In the case of the SAM trap, the number of samples available for CaCO3 and calcareous nannoplankton analyses was different, covering a period of 313 and 191 d respectively. Since gaps were quasi-equally distributed along the time series, annual fluxes were estimated by filling the gaps in the record with average fluxes calculated from the available data. The estimated range of the annual contribution of coccolithophores to total CaCO3 export at the SOTS and SAM traps was calculated by multiplying the coccolith flux of each species in each sampling interval by its average coccolith weight values obtained with the birefringence and morphometric techniques.
2.8 Remotely sensed chlorophyll a and PIC concentrations
Weekly chlorophyll a and PIC concentrations for the sampling intervals at the SOTS and SAM sites were derived from Giovanni online data system, developed and maintained by the NASA Goddard Earth Sciences Data Active Archive Center (Acker and Leptoukh, 2007). Each value is a weekly value produced by computing spatial averages within the area 48.5–45.5∘ S and 130–150∘ E for the SOTS site and 47–45∘ S and 171∘ E–179∘ W for the SAM site (Fig. 5).
3.1 Magnitude and seasonality of coccolithophore and CaCO3 fluxes
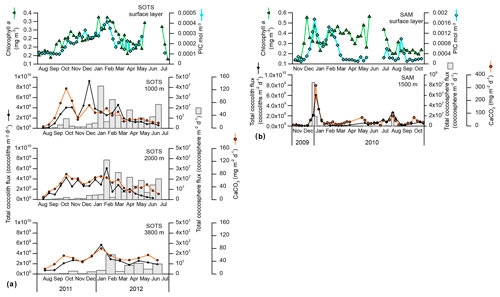
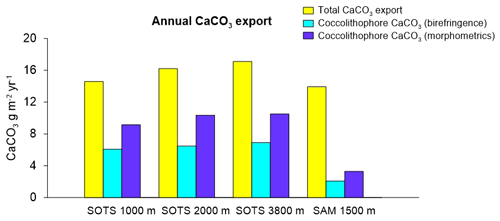
Annualized coccolith fluxes were similar at the three SOTS trap depths, with 8.6, 7.3 and 8.6×1011 coccoliths m−2 yr−1 at 1000, 2000 and 3800 m respectively, and about 3 times larger than those of the SAM site (3.0×1011 coccoliths m−2 yr−1). The contribution of intact coccospheres to the total coccolith export was low at both sites, with annual coccosphere fluxes 2 orders of magnitude lower than coccolith fluxes at SOTS (3.5, 3.3 and 1.8×109 coccospheres m−2 yr−1 at 1000, 2000 and 3800 m, respectively) and SAM (2.2×109 coccospheres m−2 yr−1). Annualized CaCO3 export was similar at both sites with 14.6, 16.2 and 17.1 g m−2 yr−1 at 1000, 2000 and 3800 m at the SOTS site and 13.9 g m−2 yr−1 at the SAM sediment trap (1500 m).
Both coccolith and coccosphere fluxes displayed a marked seasonality that followed the general trend of algal biomass accumulation in the surface waters at the SOTS and SAM sites (Fig. 2). Coccolith fluxes at 1000 m started to increase in early October and remained above the threshold of 1×109 coccoliths m−2 d−1 until mid-April (Fig. 2). Three maxima were recorded during the period of high coccolith export: October–early November 2011 (4×109 coccoliths m−2 d−1), late December 2011 (9×109 coccoliths m−2 d−1) and March 2012 (4×109 coccoliths m−2 d−1). Coccolith fluxes of the main coccolithophore species generally followed the similar seasonal pattern to that of the total coccolith flux (Fig. S1 in the Supplement) and are not discussed further. Coccolithophore fluxes registered by the 2000 and 3800 m sediment traps followed a generally similar seasonal pattern to those of the shallower trap at the SOTS site (Fig. 2). At SAM, coccolith fluxes exhibited a strong seasonality with peak fluxes in early January 2010 (up to 6×109 coccoliths m−2 d−1) and a secondary peak in August 2010 (3×109 coccoliths m−2 d−1). Coccosphere fluxes at both sites displayed maximum fluxes during the austral summer and minima during winter; however maximum coccosphere export peaks did not always match those of coccolith export (Fig. 2). The seasonality of total CaCO3 followed a similar pattern to coccolith fluxes with peak values in the spring–summer and minima during winter at both study sites.
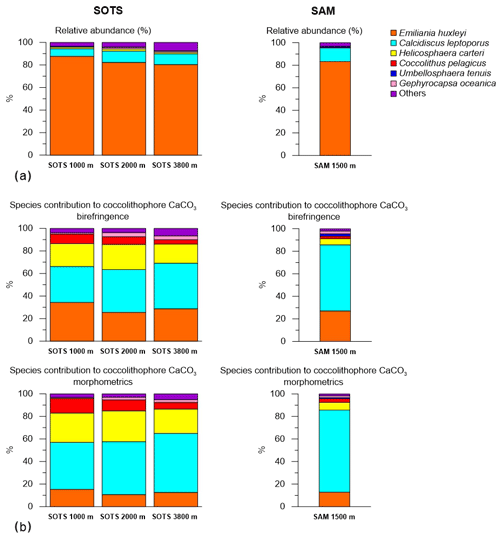
3.2 Coccolithophore assemblage composition
Coccolith sinking assemblages were overwhelmingly dominated by Emiliania huxleyi for all sediment trap records analysed (Fig. 3a). At the SOTS site, the annualized flux-weighted relative contribution of E. huxleyi decreased slightly with depth, comprising 88 % of the total coccolithophore assemblage at 1000 m, 82 % at 2000 m and 80 % at 3800 m. Secondary components of the coccolith sinking assemblage were Calcidiscus leptoporus (sensu lato) (6.8 %, 10.1 % and 9.6 % at 1000, 2000 and 3900 m, respectively), Helicosphaera carteri (1.4 %, 2 % and 1.3 %) and small Gephyrocapsa spp. (<3 µm) (1.4 %, 1.5 % and 4.7 %). Background concentrations (≤1 %) of Calciosolenia spp., Coccolithus pelagicus, Gephyrocapsa muellerae, Gephyrocapsa oceanica, Gephyrocapsa spp. (>3 µm), Syracosphaera pulchra, Syracosphaera spp., Umbellosphaera tenuis (sensu lato) and Umbilicosphaera sibogae were also registered. At the SAM site, E. huxleyi accounted for 83 % of the annualized coccolith flux, with subordinate contributions of C. leptoporus (12.2 %) and Gephyrocapsa spp. (<3 µm) (1.5 %). Background concentrations (<1 %) of Calciosolenia spp., Coccolithus pelagicus, G. oceanica, Gephyrocapsa muellerae, Gephyrocapsa spp. (>3 µm), H. carteri, Syracosphaera pulchra, Syracosphaera spp., U. sibogae and U. tenuis were observed.
3.3 Calcite content per species
Coccolith length and mass for all species measured using birefringence and morphometric techniques are provided in Table 1. Overall, the average coccolith mass estimates for the coccolithophore species at SOTS and SAM sites using both approaches are within the range of values in the published literature. The Noelaerhabdaceae family members, G. oceanica and Gephyrocapsa spp., display almost identical mass values with both approaches (Fig. 4). In contrast, substantial discrepancies are identifiable for C. pelagicus, C. leptoporus, H. carteri and U. sibogae, for which coccolith mass estimates are about 2-fold greater using morphometrics than with the birefringence approach. The range of annual contributions of coccolithophores to carbonate is illustrated in Fig. 5.
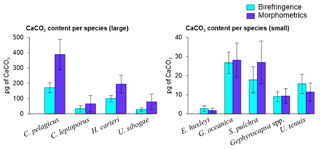
Figure 4Average and standard deviation of the coccolith mass estimates of the most important coccolithophore species captured by the SOTS and SAM sediment traps using birefringence (C-Calcita) and morphometric approaches. For E. huxleyi, the morphometrics-based coccolith mass estimate was calculated by applying a mean shape factor constant (ks) value estimated from the range of all the morphotypes found at the SAZ (i.e. ks=0.0275, Table 1).
4.1 Coccolithophore phenology in the SAZ: satellite versus sediment trap records
Total coccolith flux seasonality at the SOTS site shows good congruence with satellite-derived PIC in the surface layer, with both parameters suggesting enhanced coccolithophore productivity between October and March (austral mid-spring to early autumn; Fig. 2a). Interestingly, substantial coccosphere export ( coccospheres m−2 d−1) does not occur until January, indicating that coccolith and coccosphere export are not tightly coupled in the subantarctic waters south of Australia. Two different processes could be invoked to explain the mismatch between coccolith and coccosphere fluxes at this site. Firstly, E. huxleyi, the dominant coccolithophore species in the Southern Ocean, is able to produce coccoliths rapidly (up to three coccoliths per hour; Paasche, 1962; Balch et al., 1996) and shed the excess of coccoliths into the surrounding water under certain environmental conditions (Paasche, 2002). Although the coccolith shedding rate of E. huxleyi increases linearly with cellular growth rate (Fritz and Balch, 1996; Fritz, 1999), the tiny size and low weight of detached coccoliths allow them to remain in the upper water column long after cell numbers have begun to decline. It follows that high concentrations of detached coccoliths do not necessary imply a proportional abundance of coccospheres in the surface layer (Tyrrell and Merico, 2004; Poulton et al., 2013) or in the traps. Additionally, a substantial fraction of the coccospheres produced in the surface layer may experience substantial mechanical breakage by zooplankton before reaching the trap depths. Indeed, microzooplankton grazing pressure can remove up to 82 % of the primary production in midsummer in the subantarctic waters south of Tasmania (Ebersbach et al., 2011) and about 60 % of the daily coccolithophore growth in the North Atlantic (Mayers et al., 2019), therefore suggesting a strong top-down control on coccolithophore populations. Additionally, a polyacrylamide gel sediment trap study in the subantarctic waters south of Tasmania by Ebersbach et al. (2011) revealed that most of the particles exported out the mixed layer during the productive period occur in the form of faecal aggregates. Therefore, it is highly likely that (i) the intensity of coccosphere export registered by the traps is influenced by grazing pressure in the surface layer and (ii) the impact of grazing on coccolithophores varies throughout the year (Calbet et al., 2008; Lawerence and Menden-Deuer, 2012; Quéguiner, 2013).
In contrast, seasonal variations in satellite-derived PIC concentration and coccolith fluxes at SAM show some discrepancies not observed at SOTS. While maximum PIC concentrations in the surface layer and coccolith and coccosphere fluxes co-occur in December and January (austral early to midsummer), satellite-derived PIC suggests a secondary maximum in February–early March not recorded by the trap (Fig. 2b). One possibility is that the satellite secondary maximum is not coccoliths. The higher chlorophyll a levels at the SAM site (Fig. 2) suggest that other phytoplankton groups, such as diatoms, are more abundant than in the subantarctic waters south of Tasmania. Empty and broken diatom valves have been suggested to display similar spectral characteristics to those of coccolithophore blooms (Broerse et al., 2003; Tyrrell and Merico, 2004; Winter et al., 2014). Therefore, the second peak in satellite-derived PIC could have been caused by a senescent diatom bloom. This hypothesis is likely, since diatom blooms in the SAZ are known to develop early in the productive season (Rigual-Hernández et al., 2015b) and rapidly decay following the depletion of silicate and/or iron stocks in the surface layer (Lannuzel et al., 2011). However, no secondary late summer maximum was observed in biogenic silica fluxes in the SAM. Another possible explanation is a contribution to the satellite record from lithogenic material or storm-induced microbubble injection (Zhang et al., 2002). Fully resolving causes of mismatches between in situ and satellite PIC estimates is not achievable for the SAM site (nor more broadly for the Southern Ocean; Trull et al., 2018).
A second difference between the SAM and SOTS sites is that maximum annual coccosphere export occurred 1 week earlier than maximum coccolith fluxes at SAM (Fig. 2). The different seasonalities between the sites suggest that different export mechanisms may operate. The formation of rapidly sinking algal aggregates by diatoms is known to scavenge particles they have collided with and increase particle sinking (Alldredge and McGillivary, 1991; Passow and De La Rocha, 2006); thus, the formation of such rapidly sinking aggregates could potentially facilitate the preservation of coccospheres early in the productive season at the SAM site. However, the lack of accompanying in situ information on plankton community structure in the study region precludes the assessment of these hypotheses.
Despite the uncertainties involved in our interpretations, the overall picture that emerges from our comparison of satellite and sediment trap flux data is that the duration of the coccolithophore bloom based on ocean-colour-based PIC concentrations most likely provides an overestimation of the coccolithophore productive season. Our observations motivate caution in describing coccolithophore phenology solely based on satellite-derived PIC concentrations (e.g. Hopkins et al., 2015).
4.2 Magnitude and composition of subantarctic coccolithophore assemblages
Annual coccolith export across the major zonal systems of the Australian sector of the Southern Ocean exhibits a clear latitudinal gradient, with maximum fluxes at the SAZ (8.6×1011 coccoliths m−2 yr−1) and 8-fold lower fluxes in the polar waters of the Antarctic zone (AZ; 1.0×1011 coccoliths m−2 yr−1; Rigual Hernández et al., 2018). Coccolithophore species occurrence documented by our subantarctic sediments traps is consistent with previous reports on coccolithophore assemblage compositions in the surface layer (Findlay and Giraudeau, 2000; Saavedra-Pellitero et al., 2014; Malinverno et al., 2015; Chang and Northcote, 2016) and sediments (Findlay and Giraudeau, 2000; Saavedra-Pellitero and Baumann, 2015) and are more diverse than those found in the AZ (Rigual Hernández et al., 2018). The southward decline in coccolithophore abundance and diversity is most likely due to the decrease in sea-surface temperature (SST) and light availability moving poleward (Charalampopoulou et al., 2016; Trull et al., 2018). In particular, the close relationship between temperature and growth rates has been demonstrated in a range of coccolithophore species and strains (Buitenhuis et al., 2008) and seems to be a critical, if not the most important, control on the biogeographical distribution of coccolithophore species in the Southern Ocean (Trull et al., 2018). This pronounced latitudinal change in coccolithophore assemblage composition contrasts with the little longitudinal variability between the subantarctic SOTS and SAM sites (Fig. 3). These observations underscore the role of circumpolar fronts as natural physical barriers for plankton species distribution in the Southern Ocean (Medlin et al., 1994; Boyd, 2002; Cook et al., 2013).
Notably, the rare occurrence of the cold-water species Coccolithus pelagicus at the SOTS and SAM sites contrasts with the high contribution of C. pelagicus to the living coccolithophore communities in the subpolar and polar waters of the North Atlantic and North Pacific oceans, where it is often the second most abundant species after E. huxleyi (McIntyre and Bé, 1967; Baumann et al., 2000; Broerse et al., 2000a, b; Ziveri et al., 2000). This important difference in species composition between Northern and Southern Hemisphere subpolar ecosystems could have important implications in the calibration of the satellite PIC signal in the Southern Ocean. Previous research in the Southern Ocean comparing satellite and shipboard observations identified a substantial overestimation of coccolithophore PIC in the Southern Ocean waters by satellite ocean-colour-based PIC algorithms (Holligan et al., 2010; Trull et al., 2018). Since satellite reflectance observations are mainly calibrated against Northern Hemisphere PIC results (Balch et al., 2011, 2016), the lower calcite content of the dominant E. huxleyi morphotypes (B/C) in the Southern Ocean compared to their northern hemispheric counterparts has been suggested as a possible factor causing the overestimation of PIC concentrations in the Southern Ocean. Following this reasoning, we speculate that differences in other components of the coccolithophore assemblages, and particularly differences in C. pelagicus numbers, could contribute to the overestimation of PIC concentrations by the satellite PIC algorithm in the Southern Ocean. Indeed, the scaling of reflectance (in satellite images) to PIC (in ocean) is very dependent on coccolith area : mass ratios (Gordon and Du, 2001; Balch et al., 2005a). Coccolithus pelagicus has remarkably heavier and thicker coccoliths (100–400 pg per coccolith; Table 1) than E. huxleyi (∼3 pg per coccolith), i.e. about 100 times heavier. However, the average coccolith area of C. pelagicus is only about 10 times greater than that of E. huxleyi. Thus, this lack of a proportional relationship between area and mass between these species should be taken into consideration when calibrating the satellite signals of coccolithophore-related PIC in the Southern Ocean. However, it should be noted that this is only one possible factor contributing to the overestimation of PIC concentrations in Southern Ocean waters. Other factors such as the presence of microbubbles – which are a source of enhanced reflectance – must also play an important role (Balch et al., 2011).
4.3 Coccolith calcite content of subantarctic coccolithophore species
Multiple methodological biases associated with each of the methods used for estimating coccolith calcite content (i.e. birefringence, morphometrics) could be invoked to explain the different estimates observed for some of the species (see Young and Ziveri, 2000; Fuertes et al., 2014, and references therein). However, the fact that these discrepancies vary greatly across species suggests that the composition of the crystal units of the coccoliths could be the most important factor causing these differences. All the heterococcoliths of the species analysed are mainly composed of either V- or R-calcite crystal units or a combination of both (Young et al., 2003; Table 1). R units are characterized by sub-radial c axes that are reasonably well measured by the birefringence technique, but the almost vertical optical axes of the V units (Young, 1992; Young et al., 1999) make the same thickness less birefringent (Fuertes et al., 2014). Thus, it is likely that differences in the birefringence properties of the R and V units could be responsible for the different estimates provided by the two approaches. This is supported by our results, which show coccolith mass estimates of those species composed of R units, such as G. oceanica and Gephyrocapsa spp., exhibit almost identical values with both techniques (Table 1). In contrast, those species with coccoliths composed by a combination of R and V units, such as C. pelagicus, C. leptoporus, H. carteri and U. sibogae, display divergent mass estimates between approaches. The case of E. huxleyi is more complex due to the large intraspecific genetic variability that results in substantial differences in the profile and degree of calcification between specimens (Young and Ziveri, 2000). Our birefringence mass estimate for E. huxleyi (2.67±1.49 pg) is less than 1 pg lower than the mean range value calculated with the morphometric technique (i.e. 1.81±1.10 pg with an average ks value of all the morphotypes found at the SAZ; i.e. ks=0.0275) but identical to the maximum (2.64±1.60 pg; using ks=0.04). These results suggest a reasonably good consistency between techniques for E. huxleyi.
Taking into consideration all the above, it is likely that the coccolith mass of some species is underestimated by the birefringence technique, and therefore the fractional contribution of coccolithophores to total PIC using this approach should be taken as a conservative estimate. Since both methods for estimating calcite content have inherent uncertainties, the range of values provided by both techniques is used here as an approximation of the fractional contribution of coccolithophores to total annual CaCO3 export to the deep sea in the Australian and New Zealand sectors of the SAZ.
4.4 Contribution of coccolithophores to carbonate export in the Australian–New Zealand sectors of the Southern Ocean
The magnitude of the total PIC export in the subantarctic waters was similar between the SOTS and SAM sites (range 14–17 g m−2 yr−1) and thus slightly above the global average (11 g m−2 yr−1; Honjo et al., 2008). Our estimates indicate that coccolithophores are major contributors to CaCO3 export in the Australian and New Zealand waters of the SAZ, accounting for 40 %–60 % and 15 %–25 % of the annual CaCO3 export, respectively (Fig. 5). Heterotrophic calcifiers, mainly planktonic foraminifera (Salter et al., 2014), must therefore account for the remainder of the annual CaCO3 export at both sites. Previous work on foraminifera fluxes in our study regions allows for an approximate estimate of the contribution of foraminifera to total CaCO3 flux that can be used to assess the validity of our estimates. Combining counts of foraminifera shells (King and Howard, 2003) with estimates of their average shell weights (20–40 µg per shell depending on size; Moy et al., 2009) suggests contributions of one-third to two-thirds of planktonic foraminifera to the total CaCO3 flux in the Australian SAZ (Trull et al., 2018). In the subantarctic waters south of New Zealand, Northcote and Neil (2005) estimated that planktonic foraminifera accounted for about 78 %–97 % of the total CaCO3. Thus, estimations of the contribution of heterotrophic calcifiers to total carbonate in both study regions are in reasonable agreement with our coccolithophore CaCO3 estimates at both sites. The lower contribution of coccolithophores to CaCO3 export at the SAM site in comparison with that of SOTS may be explained by differences in the ecosystem structure between sites. Algal biomass accumulation in the surface waters of the SAM region (average chlorophyll a concentration between 2002 and 2018 is 0.31 mg m−3) is substantially higher than that at SOTS (0.23 mg m−3). We speculate that the higher abundance of non-calcareous phytoplankton (e.g. diatoms) in the subantarctic waters south of New Zealand could simultaneously reduce coccolithophore biomass through resource competition (Le Quéré et al., 2005; Sinha et al., 2010) while stimulating foraminifera growth (Schiebel et al., 2017). The combination of both factors could be responsible for the lower coccolithophore productivity at the SAM site despite similar total CaCO3 export. Assuming that both the SOTS and SAM sites can be considered representative of a broad longitudinal swath of the SAZ south of Australia and New Zealand (ca. 1 % of areal extent of the global ocean), the coccolithophore CaCO3 export in these two regions together accounts for approximately 0.4 Tmol Cinorg yr−1. This value represents approximately 1 % of the global annual PIC export to the deep ocean (Honjo et al., 2008) and underscores the notion that the high-nutrient, low-chlorophyll waters of the circumpolar SAZ should not be taken as indicative of low biological activity or export.
Our results indicate that although E. huxleyi overwhelmingly dominates the coccolithophore sinking assemblages at both study sites, other species with lower relative contribution but substantially heavier coccoliths are more important contributors to the annual coccolithophore CaCO3 export budget (Fig. 3). Particularly relevant is the case of C. leptoporus, which despite its relatively low abundance (∼ 10 % of the annual assemblage at both sites; Fig. 3) accounts for between 30 %–50 % and 60 %–70 % of the annual coccolithophore CaCO3 export at the SOTS and SAM sites, respectively (Fig. 3). Similarly, other species with heavy coccoliths, such as H. carteri and C. pelagicus, are important contributors to the annual coccolithophore PIC export to the deep sea (up to ∼30 % and ∼10 % of the annual coccolithophore PIC, respectively) despite their low annual relative abundance (<2 % at both sites) (Fig. 3). These results serve as an important reminder that it is often not the most abundant species but rather the largest coccolithophore species that account for the greatest contribution to coccolithophore CaCO3 production and export (Young and Ziveri, 2000; Baumann et al., 2004; Daniels et al., 2016).
The important contribution made by the coccolithophore community in setting the magnitude of carbonate production and export to the deep sea is evidenced when we compare the coccolith and total CaCO3 fluxes of the SOTS sediment trap with those deployed in the AZ along the 140∘ E meridian (Fig. 1). Although both total and coccolithophore CaCO3 export decrease with increasing latitude, these changes are largely uneven. While total CaCO3 decreases 2-fold from the SAZ to the AZ, coccolithophore CaCO3 export decreases 28-fold (Fig. S2). This lack of proportional latitudinal change can be attributed to two main factors. First, subantarctic coccolithophore populations are diverse and relatively rich in species with large and heavy coccoliths such as C. leptoporus or H. carteri that account for a large fraction of the annual carbonate production and export. South of the PF, assemblages become monospecific, or nearly monospecific, dominated by the small and relatively lightly calcified E. huxleyi. Second, latitudinal variations in the abundance of heterotrophic calcifiers (mainly foraminifera but also pteropods) must play a major role in modulating the observed variations in CaCO3 export. In particular, our data suggest that the fractional contribution of heterotrophic calcifiers to CaCO3 production increases from ∼ 40 %–60 % in the Australian SAZ to up to 95 % in the AZ (Rigual Hernández et al., 2018). This pattern is consistent with previous shipboard and sediment trap studies that reported higher abundances of planktonic foraminifera at the PFZ and AZ compared to that of the SAZ in the Australian sector (King and Howard, 2003; Trull et al., 2018). Controls on the biogeographic distribution of foraminifera species are complex and beyond the scope of this paper; however, we provide a few observations. Both temperature and diet are critical factors controlling the spatial distribution of planktonic foraminifera species. In particular, the lower temperatures south of the SAF seem to favour the development of Neogloboquadrina pachyderma sin. and Turborotalita quinqueloba as indicated by the high abundance of these species in the PFZ (>80 % of the annual integrated flux for both species together; King and Howard, 2003). Additionally, the dramatically different algal communities dwelling in each zonal system may also play a role in planktonic foraminifera species distributions. In particular, diatoms can account for a major part of the diet of some foraminifera species, including N. pachyderma (Schiebel and Hemleben, 2017). Therefore, it is likely that the preferential grazing on diatoms of some foraminifera species may play an important role in the increase in foraminifera CaCO3 production moving poleward.
4.5 Future predictions of coccolithophore community response to environmental change in the subantarctic zone
The response of E. huxleyi to environmental change has been extensively studied in laboratory experiments (Meyer and Riebesell, 2015; Müller et al., 2015; Feng et al., 2017), and the available information is sufficient to propose possible changes of its niche and calcification in the Southern Ocean, as discussed in detail in Trull et al. (2018) and Krumhardt et al. (2017). Due to the ubiquity and abundance of E. huxleyi, the ecophysiology of this species is often regarded as typical of all coccolithophores. However, E. huxleyi is rather different from most other coccolithophore species in that its physiological adaptations place it in the upper limit of the r−K ecological gradient of these organisms (i.e. an opportunistic species), while most of the other species are located at the opposite end of the spectrum (i.e. conservative or K-selected species) (Probert and Houdan, 2004). Our results demonstrate that E. huxleyi plays an important but not dominant role in CaCO3 export, with other species such as C. leptoporus, H. carteri or C. pelagicus making a larger contribution to the annual CaCO3 export in the SAZ (Fig. 3). Therefore, it is of critical importance to evaluate how these other biogeochemically important coccolithophore species will respond to projected climate-induced changes in the Southern Ocean. Here, we now assess the response of large coccolithophore species to projected changes in temperature and carbonate chemistry that have been highlighted among the most important environmental stressors expected to impact Southern Ocean coccolithophore physiological rates (Müller et al., 2015; Charalampopoulou et al., 2016; Feng et al., 2017; Trull et al., 2018).
The Southern Ocean is warming rapidly (Gille, 2002; Böning et al., 2008), largely due to the southward migration of the Antarctic Circumpolar Current (ACC) fronts (Sokolov and Rintoul, 2009). Only between 1992 and 2007 the position of Southern Ocean fronts shifted by approximately 60 km to the south (Sokolov and Rintoul, 2009), and this trend may continue throughout the next century. Therefore, it is likely that any further southward migration of ACC fronts will be coupled with an expansion of subantarctic coccolithophore species towards higher latitudes. The poleward expansion of the E. huxleyi geographic range has already been suggested in the Southern Ocean (Cubillos et al., 2007; Winter et al., 2014; Charalampopoulou et al., 2016), and it also appears to be occurring in the North Atlantic (Rivero-Calle et al., 2015; Neukermans et al., 2018). Given the important contribution of large subantarctic coccolithophore species to CaCO3 export, the expansion of their ecological niche could result in a substantial increase in CaCO3 production and export in the Southern Ocean. However, this may not be the future scenario for the SAZ southeast on New Zealand, where bathymetry strongly controls the location of ocean fronts (Fernandez et al., 2014; Chiswell et al., 2015). If the fronts are bathymetrically “locked”, then the SAZ will not expand in areal extent, although the region is still predicted to undergo significant physical, biogeochemical and biological changes (Law et al., 2018) that will have likely flow-on effects on coccolithophore productivity and export (Deppeler and Davidson, 2017).
The available carbonate chemistry manipulation experiments with C. leptoporus have come to different conclusions. While some studies identified an increase in coccolith malformations with increasing CO2 concentrations (Langer et al., 2006; Langer and Bode, 2011; Diner et al., 2015), another study (Fiorini et al., 2011) reported no changes in the calcification of C. leptoporus at elevated pCO2. Interestingly, C. leptoporus did not experience changes in its photosynthesis rates over the tested CO2 range in any of the aforementioned studies. The most likely explanation for the different results between the studies is a strain-specific variable response to changing carbonate chemistry (Diner et al., 2015). Strain-specific variability in response to changing carbonate chemistry has been previously reported in other coccolithophores, such as E. huxleyi (Langer et al., 2009; Müller et al., 2015), and therefore it is likely that this also occurs in other species. Given the fact that Southern Ocean fronts act as barriers for species distributions and gene flows (Medlin et al., 1994; Patarnello et al., 1996; Thornhill et al., 2008; Cook et al., 2013), it is possible that the subantarctic C. leptoporus populations exhibit a different ecophysiology than those used in the above-mentioned laboratory experiments. Prediction of the responses of H. carteri and C. pelagicus is even more challenging due to the lack of experiments testing the response of these species to changing seawater carbonate chemistry. The only available insights in the response of one of these species to ocean acidification are found in the fossil record. Both Gibbs et al. (2013) and O'Dea et al. (2014) reconstructed the evolution of C. pelagicus populations during the Palaeocene-Eocene Thermal Maximum (PETM), a period arguably regarded as the best geological approximation of the present rapid rise in atmospheric CO2 levels and temperatures. These studies concluded that C. pelagicus most likely reduced its growth rates and calcification during this period. This limited number of studies suggests that the ongoing ocean acidification in the Southern Ocean could potentially have a negative impact on the physiological rates of C. leptoporus and C. pelagicus, while the effect on H. carteri is unknown. Physiological response experiments (e.g. Müller et al., 2015) with Southern Ocean strains of C. leptoporus, H. carteri and C. pelagicus are, therefore, urgently needed to be able to quantify the effect of projected changes in oceanic conditions in the SAZ on their physiological rates and consequent effects on carbon cycling in the Southern Ocean.
Our synthesis suggests opposing influence of environmental stressors on subantarctic coccolithophore populations. Poleward migration of fronts will likely increase coccolithophore CaCO3 production in the Southern Ocean, while changes in carbonate chemistry speciation will reduce growth rates of subantarctic coccolithophores. It seems possible that coccolithophores will initially expand southward as waters warm and fronts migrate but then eventually diminish as acidification overwhelms those changes.
Morphometric data of major coccolithophore species generated during the current study are listed in Table 1, while species relative abundance and species fluxes (plotted in Fig. S1) can be accessed via the following link: https://doi.org/10.26179/5ddf3db06a153 (Rigual-Hernandez et al., 2019).
The supplement related to this article is available online at: https://doi.org/10.5194/bg-17-245-2020-supplement.
TWT, SDN, DMD and LCN planned and performed the field experiment. ASRH led the coccolithophore study and performed the sample processing as well as the microscopy and image analyses. AMB and ASRH performed the SEM analyses. ASRH and SDN performed the numerical analyses. ASRH wrote the paper with feedback from all authors.
The authors declare that they have no conflict of interest.
The SOTS mooring work was supported by IMOS, the ACE CRC and the Australian Marine National Facility. The work at SAM was supported by funding provided by the New Zealand Ministry of Business, Innovation and Employment and previous agencies, and most recently by NIWA's Strategic Science Investment Fund. NIWA is acknowledged for providing capital grants for mooring equipment purchases, and we thank all the NIWA scientists, technicians and vessel staff, who participated in the New Zealand biophysical moorings programme (2000–2012). Cathryn Wynn-Edwards (IMAS) provided support in sample splitting/processing and laboratory analysis. Satellite chlorophyll a and PIC data sets were produced with the Giovanni online data system, developed and maintained by the NASA GES DISC. We thank Griet Neukermans and Alex Poulton for their constructive comments and suggestions that helped improve and clarify this paper.
This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement number 748690 – SONAR-CO2 (Andrés S. Rigual Hernández, José A. Flores and Fátima Abrantes).
This paper was edited by Jean-Pierre Gattuso and reviewed by Griet Neukermans and Alex Poulton.
Acker, J. G. and Leptoukh, G.: Online Analysis Enhances Use of NASA Earth Science Data, EOS T. Am. Geophys. Un., 88, 14–17, 2007.
Alldredge, A. L. and McGillivary, P.: The attachment probabilities of marine snow and their implications for particle coagulation in the ocean, Deep-Sea Res. Pt A, 38, 431–443, https://doi.org/10.1016/0198-0149(91)90045-H, 1991.
Alvain, S., Le Quéré, C., Bopp, L., Racault, M.-F., Beaugrand, G., Dessailly, D., and Buitenhuis, E. T.: Rapid climatic driven shifts of diatoms at high latitudes, Remote Sens. Environ., 132, 195–201, https://doi.org/10.1016/j.rse.2013.01.014, 2013.
Bach, L. T., Riebesell, U., Gutowska, M. A., Federwisch, L., and Schulz, K. G.: A unifying concept of coccolithophore sensitivity to changing carbonate chemistry embedded in an ecological framework, Prog. Oceanogr., 135, 125–138, https://doi.org/10.1016/j.pocean.2015.04.012, 2015.
Bairbakhish, A. N., Bollmann, J., Sprengel, C., and Thierstein, H. R.: Disintegration of aggregates and coccospheres in sediment trap samples, Mar. Micropaleontol., 37, 219–223, https://doi.org/10.1016/S0377-8398(99)00019-5, 1999.
Balch, W. M., Fritz, J., and Fernandez, E.: Decoupling of calcification and photosynthesis in the coccolithophore Emiliania huxleyi under steady-state light-limited growth, Mar. Ecol.-Prog. Ser., 142, 87–97, 1996.
Balch, W. M., Gordon, H. R., Bowler, B. C., Drapeau, D. T., and Booth, E. S.: Calcium carbonate measurements in the surface global ocean based on Moderate-Resolution Imaging Spectroradiometer data, J. Geophys. Res.-Oceans, 110, https://doi.org/10.1029/2004JC002560, 2005a.
Balch, W. M., Gordon, H. R., Bowler, B. C., Drapeau, D. T., and Booth, E. S.: Calcium carbonate measurements in the surface global ocean based on Moderate-Resolution Imaging Spectroradiometer data, J. Geophys. Res.-Oceans, 110, C07001, https://doi.org/10.1029/2004JC002560, 2005b.
Balch, W. M., Drapeau, D. T., Bowler, B. C., Lyczskowski, E., Booth, E. S., and Alley, D.: The contribution of coccolithophores to the optical and inorganic carbon budgets during the Southern Ocean Gas Exchange Experiment: New evidence in support of the “Great Calcite Belt” hypothesis, J. Geophys. Res.-Oceans, 116, C00F06, https://doi.org/10.1029/2011JC006941, 2011.
Balch, W. M., Bates, N. R., Lam, P. J., Twining, B. S., Rosengard, S. Z., Bowler, B. C., Drapeau, D. T., Garley, R., Lubelczyk, L. C., Mitchell, C., and Rauschenberg, S.: Factors regulating the Great Calcite Belt in the Southern Ocean and its biogeochemical significance, Global Biogeochem. Cy., 30, 1124–1144, https://doi.org/10.1002/2016GB005414, 2016.
Baumann, K. H., Andruleit, H., and Samtleben, C.: Coccolithophores in the Nordic Seas: comparison of living communities with surface sediment assemblages, Deep-Sea Res. Pt. II, 47, 1743–1772, https://doi.org/10.1016/S0967-0645(00)00005-9, 2000.
Baumann, K.-H., Böckel, B., and Frenz, M.: Coccolith contribution to South Atlantic carbonate sedimentation, in: Coccolithophores: From Molecular Processes to Global Impact, edited by: Thierstein, H. R. and Young, J. R., Springer Berlin Heidelberg, Berlin, Heidelberg, 367–402, 2004.
Beaufort, L.: Weight estimates of coccoliths using the optical properties (birefringence) of calcite, Micropaleontology, 51, 289–297, https://doi.org/10.2113/gsmicropal.51.4.289, 2005.
Beaufort, L. and Heussner, S.: Coccolithophorids on the continental slope of the Bay of Biscay – production, transport and contribution to mass fluxes, Deep-Sea Res. Pt. II, 46, 2147–2174, https://doi.org/10.1016/S0967-0645(99)00058-2, 1999.
Beaufort, L., Barbarin, N., and Gally, Y.: Optical measurements to determine the thickness of calcite crystals and the mass of thin carbonate particles such as coccoliths, Nat. Protoc., 9, 633, https://doi.org/10.1038/nprot.2014.028, 2014.
Bijma, J., Hönisch, B., and Zeebe, R. E.: Impact of the ocean carbonate chemistry on living foraminiferal shell weight: Comment on “Carbonate ion concentration in glacial-age deep waters of the Caribbean Sea” by W. S. Broecker and E. Clark, Geochem. Geophy. Geosy., 3, 1–7, https://doi.org/10.1029/2002GC000388, 2002.
Bolton, C. T., Hernandez-Sanchez, M. T., Fuertes, M.-A., Gonzalez-Lemos, S., Abrevaya, L., Mendez-Vicente, A., Flores, J.-A., Probert, I., Giosan, L., Johnson, J., and Stoll, H. M.: Decrease in coccolithophore calcification and CO2 since the middle Miocene, Nat. Commun., 7, 10284, https://doi.org/10.1038/ncomms10284, 2016.
Böning, C. W., Dispert, A., Visbeck, M., Rintoul, S. R., and Schwarzkopf, F. U.: The response of the Antarctic Circumpolar Current to recent climate change, Nat. Geosci., 1, 864, https://doi.org/10.1038/ngeo362, 2008.
Bowie, A. R., Brian Griffiths, F., Dehairs, F., and Trull, T.: Oceanography of the subantarctic and Polar Frontal Zones south of Australia during summer: Setting for the SAZ-Sense study, Deep-Sea Res. Pt. II, 58, 2059–2070, https://doi.org/10.1016/j.dsr2.2011.05.033, 2011.
Boyd, P. W.: Environmental factors controlling phytoplankton processes in the Southern Ocean, J. Phycol., 38, 844–861, https://doi.org/10.1046/j.1529-8817.2002.t01-1-01203.x, 2002.
Boyd, P. W. and Trull, T. W.: Understanding the export of biogenic particles in oceanic waters: Is there consensus?, Prog. Oceanogr., 72, 276–312, https://doi.org/10.1016/j.pocean.2006.10.007, 2007.
Broerse, A. T. C., Ziveri, P., and Honjo, S.: Coccolithophore (-CaCO3) flux in the Sea of Okhotsk: seasonality, settling and alteration processes, Mar. Micropaleontol., 39, 179–200, https://doi.org/10.1016/S0377-8398(00)00020-7, 2000a.
Broerse, A. T. C., Ziveri, P., van Hinte, J. E., and Honjo, S.: Coccolithophore export production, species composition, and coccolith-CaCO3 fluxes in the NE Atlantic (34∘ N 21∘ W and 48∘ N 21∘ W), Deep-Sea Res. Pt. II, 47, 1877–1905, https://doi.org/10.1016/S0967-0645(00)00010-2, 2000b.
Broerse, A. T. C., Tyrrell, T., Young, J. R., Poulton, A. J., Merico, A., Balch, W. M., and Miller, P. I.: The cause of bright waters in the Bering Sea in winter, Cont. Shelf Res., 23, 1579–1596, https://doi.org/10.1016/j.csr.2003.07.001, 2003.
Buitenhuis, E. T., Wal, P., and Baar, H. J. W.: Blooms of Emiliania huxleyi are sinks of atmospheric carbon dioxide: A field and mesocosm study derived simulation, Global Biogeochem. Cy., 15, 577–587, https://doi.org/10.1029/2000GB001292, 2001.
Buitenhuis, E. T., Pangerc, T., Franklin, D. J., Le Quéré, C., and Malin, G.: Growth rates of six coccolithophorid strains as a function of temperature, Limnol. Oceanogr., 53, 1181–1185, https://doi.org/10.4319/lo.2008.53.3.1181, 2008.
Calbet, A., Trepat, I., Almeda, R., Saló, V., Saiz, E., Movilla, J. I., Alcaraz, M., Yebra, L., and Simó, R.: Impact of micro- and nanograzers on phytoplankton assessed by standard and size-fractionated dilution grazing experiments, Aquat. Microb. Ecol., 50, 145–156, 2008.
Cao, L. and Caldeira, K.: Atmospheric CO2 stabilization and ocean acidification, Geophys. Res. Lett., 35, L19609, https://doi.org/10.1029/2008GL035072, 2008.
Chang, F. H. and Gall, M.: Phytoplankton assemblages and photosynthetic pigments during winter and spring in the Subtropical Convergence region near New Zealand, New Zeal. J. Mar. Fresh., 32, 515–530, https://doi.org/10.1080/00288330.1998.9516840, 1998.
Chang, F. H. and Northcote, L.: Species composition of extant coccolithophores including twenty six new records from the southwest Pacific near New Zealand, Marine Biodiversity Records, 9, 75, https://doi.org/10.1186/s41200-016-0077-7, 2016.
Charalampopoulou, A., Poulton, A. J., Bakker, D. C. E., Lucas, M. I., Stinchcombe, M. C., and Tyrrell, T.: Environmental drivers of coccolithophore abundance and calcification across Drake Passage (Southern Ocean), Biogeosciences, 13, 5917–5935, https://doi.org/10.5194/bg-13-5917-2016, 2016.
Chiswell, S. M., Bostock, H. C., Sutton, P. J. H., and Williams, M. J. M.: Physical oceanography of the deep seas around New Zealand: a review, New Zeal. J. Mar. Fresh., 49, 286–317, https://doi.org/10.1080/00288330.2014.992918, 2015.
Cook, S. S., Jones, R. C., Vaillancourt, R. E., and Hallegraeff, G. M.: Genetic differentiation among Australian and Southern Ocean populations of the ubiquitous coccolithophore Emiliania huxleyi (Haptophyta), Phycologia, 52, 368–374, https://doi.org/10.2216/12-111.1, 2013.
Cubillos, J., Wright, S., Nash, G., De Salas, M., Griffiths, B., Tilbrook, B., Poisson, A., and Hallegraeff, G.: Calcification morphotypes of the coccolithophorid Emiliania huxleyi in the Southern Ocean: changes in 2001 to 2006 compared to historical data, Mar. Ecol.-Prog. Ser., 348, 47–54, 2007.
D'Amario, B., Ziveri, P., Grelaud, M., and Oviedo, A.: Emiliania huxleyi coccolith calcite mass modulation by morphological changes and ecology in the Mediterranean Sea, PLOS ONE, 13, e0201161, https://doi.org/10.1371/journal.pone.0201161, 2018.
Daniels, C. J., Poulton, A. J., Young, J. R., Esposito, M., Humphreys, M. P., Ribas-Ribas, M., Tynan, E., and Tyrrell, T.: Species-specific calcite production reveals Coccolithus pelagicus as the key calcifier in the Arctic Ocean, Mar. Ecol.-Prog. Ser., 555, 29–47, 2016.
de Salas, M. F., Eriksen, R., Davidson, A. T., and Wright, S. W.: Protistan communities in the Australian sector of the Sub-Antarctic Zone during SAZ-Sense, Deep-Sea Res. Pt. II, 58, 2135–2149, https://doi.org/10.1016/j.dsr2.2011.05.032, 2011.
Deppeler, S. L. and Davidson, A. T.: Southern Ocean Phytoplankton in a Changing Climate, Front. Mar. Sci., 4, 40, https://doi.org/10.3389/fmars.2017.00040, 2017.
Diner, R. E., Benner, I., Passow, U., Komada, T., Carpenter, E. J., and Stillman, J. H. J. M. B.: Negative effects of ocean acidification on calcification vary within the coccolithophore genus Calcidiscus, Mar. Biol., 162, 1287–1305, https://doi.org/10.1007/s00227-015-2669-x, 2015.
Dugdale, R. C., Wilkerson, F. P., and Minas, H. J.: The role of a silicate pump in driving new production, Deep-Sea Res. Pt. I, 42, 697–719, https://doi.org/10.1016/0967-0637(95)00015-X, 1995.
Ebersbach, F., Trull, T. W., Davies, D. M., and Bray, S. G.: Controls on mesopelagic particle fluxes in the Sub-Antarctic and Polar Frontal Zones in the Southern Ocean south of Australia in summer – Perspectives from free-drifting sediment traps, Deep-Sea Res. Pt. II, 58, 2260–2276, https://doi.org/10.1016/j.dsr2.2011.05.025, 2011.
Eriksen, R., Trull, T. W., Davies, D., Jansen, P., Davidson, A. T., Westwood, K., and van den Enden, R.: Seasonal succession of phytoplankton community structure from autonomous sampling at the Australian Southern Ocean Time Series (SOTS) observatory, Mar. Ecol.-Prog. Ser., 589, 13–31, 2018.
Fabry, V. J., Seibel, B. A., Feely, R. A., and Orr, J. C.: Impacts of ocean acidification on marine fauna and ecosystem processes, ICES J. Mar. Sci., 65, 414–432, https://doi.org/10.1093/icesjms/fsn048, 2008.
Fabry, V. J., McClintock, J. B., Mathis, J. T., and Grebmeier, J. M.: Ocean acidification at high latitudes: the bellweather, Oceanography, 22, 160, 2009.
Feng, Y., Roleda, M. Y., Armstrong, E., Boyd, P. W., and Hurd, C. L.: Environmental controls on the growth, photosynthetic and calcification rates of a Southern Hemisphere strain of the coccolithophore Emiliania huxleyi, Limnol. Oceanogr., 62, 519–540, https://doi.org/10.1002/lno.10442, 2017.
Fernandez, D., Bowen, M., and Carter, L.: Intensification and variability of the confluence of subtropical and subantarctic boundary currents east of New Zealand, J. Geophys. Res.-Oceans, 119, 1146–1160, https://doi.org/10.1002/2013jc009153, 2014.
Findlay, C. S. and Giraudeau, J.: Extant calcareous nannoplankton in the Australian Sector of the Southern Ocean (austral summers 1994 and 1995), Mar. Micropaleontol., 40, 417–439, https://doi.org/10.1016/S0377-8398(00)00046-3, 2000.
Fiorini, S., Middelburg, J. J., and Gattuso, J.-P.: Testing the effects of elevated pCO2 on coccolithophores (Prymnesiophyceae): comparison between haploid and diploid life stages, J. Phycol., 47, 1281–1291, https://doi.org/10.1111/j.1529-8817.2011.01080.x, 2011.
Flores, J. A. and Sierro, F. J.: A revised technique for the calculation of calcareous nannofossil accumulation rates, Micropaleontology, 43, 321–324, 1997.
Fritz, J. J.: Carbon fixation and coccolith detachment in the coccolithophore Emiliania huxleyi in nitrate-limited cyclostats, Mar. Biol., 133, 509–518, https://doi.org/10.1007/s002270050491, 1999.
Fritz, J. J. and Balch, W. M.: A light-limited continuous culture study of Emiliania huxleyi: determination of coccolith detachment and its relevance to cell sinking, J. Exp. Mar. Biol. Ecol., 207, 127–147, https://doi.org/10.1016/S0022-0981(96)02633-0, 1996.
Fuertes, M.-Á., Flores, J.-A., and Sierro, F. J.: The use of circularly polarized light for biometry, identification and estimation of mass of coccoliths, Mar. Micropaleontol., 113, 44–55, https://doi.org/10.1016/j.marmicro.2014.08.007, 2014.
Gattuso, J.-P. and Hansson, L.: Ocean acidification, Oxford University Press, Oxford, 2011.
Gibbs, S. J., Poulton, A. J., Bown, P. R., Daniels, C. J., Hopkins, J., Young, J. R., Jones, H. L., Thiemann, G. J., O'Dea, S. A., and Newsam, C.: Species-specific growth response of coccolithophores to Palaeocene–Eocene environmental change, Nat. Geosci., 6, 218, https://doi.org/10.1038/ngeo1719, 2013.
Gille, S. T.: Warming of the Southern Ocean Since the 1950s, Science, 295, 1275–1277, https://doi.org/10.1126/science.1065863, 2002.
González-Lemos, S., Guitián, J., Fuertes, M.-Á., Flores, J.-A., and Stoll, H. M.: Technical note: An empirical method for absolute calibration of coccolith thickness, Biogeosciences, 15, 1079–1091, https://doi.org/10.5194/bg-15-1079-2018, 2018.
Gordon, H. R. and Du, T.: Light scattering by nonspherical particles: Application to coccoliths detached from Emiliania huxleyi, Limnol. Oceanogr., 46, 1438–1454, https://doi.org/10.4319/lo.2001.46.6.1438, 2001.
Gordon, H. R., Boynton, G. C., Balch, W. M., Groom, S. B., Harbour, D. S., and Smyth, T. J.: Retrieval of coccolithophore calcite concentration from SeaWiFS Imagery, Geophys. Res. Lett., 28, 1587–1590, https://doi.org/10.1029/2000gl012025, 2001.
Gravalosa, J. M., Flores, J.-A., Sierro, F. J., and Gersonde, R.: Sea surface distribution of coccolithophores in the eastern Pacific sector of the Southern Ocean (Bellingshausen and Amundsen Seas) during the late austral summer of 2001, Mar. Micropaleontol., 69, 16–25, https://doi.org/10.1016/j.marmicro.2007.11.006, 2008.
Herraiz-Borreguero, L. and Rintoul, S. R.: Regional circulation and its impact on upper ocean variability south of Tasmania, Deep-Sea Res. Pt. II, 58, 2071–2081, https://doi.org/10.1016/j.dsr2.2011.05.022, 2011.
Holligan, P. M., Charalampopoulou, A., and Hutson, R.: Seasonal distributions of the coccolithophore, Emiliania huxleyi, and of particulate inorganic carbon in surface waters of the Scotia Sea, J. Marine Syst., 82, 195–205, https://doi.org/10.1016/j.jmarsys.2010.05.007, 2010.
Honjo, S., Manganini, S. J., Krishfield, R. A., and Francois, R.: Particulate organic carbon fluxes to the ocean interior and factors controlling the biological pump: A synthesis of global sediment trap programs since 1983, Prog. Oceanogr., 76, 217–285, https://doi.org/10.1016/j.pocean.2007.11.003, 2008.
Hopkins, J., Henson, S. A., Painter, S. C., Tyrrell, T., and Poulton, A. J.: Phenological characteristics of global coccolithophore blooms, Global Biogeochem. Cy., 29, 239–253, https://doi.org/10.1002/2014GB004919, 2015.
IPCC: Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (AR5), IPCC, New York, 2013.
King, A. L. and Howard, W. R.: Planktonic foraminiferal flux seasonality in Subantarctic sediment traps: A test for paleoclimate reconstructions, Paleoceanography, 18, 1019, https://doi.org/10.1029/2002pa000839, 2003.
Kopczynska, E. E., Dehairs, F., Elskens, M., and Wright, S.: Phytoplankton and microzooplankton variability between the Subtropical and Polar Fronts south of Australia: Thriving under regenerative and new production in late summer, J. Geophys. Res.-Oceans, 106, 31597–31609, https://doi.org/10.1029/2000JC000278, 2001.
Krumhardt, K. M., Lovenduski, N. S., Iglesias-Rodriguez, M. D., and Kleypas, J. A.: Coccolithophore growth and calcification in a changing ocean, Prog. Oceanogr., 159, 276–295, https://doi.org/10.1016/j.pocean.2017.10.007, 2017.
Langdon, C. and Atkinson, M. J.: Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment, J. Geophys. Res.-Oceans, 110, C09S07, https://doi.org/10.1029/2004JC002576, 2005.
Langer, G. and Bode, M. J. G.: CO2 mediation of adverse effects of seawater acidification in Calcidiscus leptoporus, Geochem. Geophy. Geosy., 12, 2011.
Langer, G., Geisen, M., Baumann, K.-H., Kläs, J., Riebesell, U., Thoms, S., and Young, J. R.: Species-specific responses of calcifying algae to changing seawater carbonate chemistry, Geochem. Geophy. Geosy., 7, Q09006, https://doi.org/10.1029/2005GC001227, 2006.
Langer, G., Nehrke, G., Probert, I., Ly, J., and Ziveri, P.: Strain-specific responses of Emiliania huxleyi to changing seawater carbonate chemistry, Biogeosciences, 6, 2637–2646, https://doi.org/10.5194/bg-6-2637-2009, 2009.
Lannuzel, D., Bowie, A. R., Remenyi, T., Lam, P., Townsend, A., Ibisanmi, E., Butler, E., Wagener, T., and Schoemann, V.: Distributions of dissolved and particulate iron in the sub-Antarctic and Polar Frontal Southern Ocean (Australian sector), Deep-Sea Res. Pt. II, 58, 2094–2112, https://doi.org/10.1016/j.dsr2.2011.05.027, 2011.
Law, C. S., Schwarz, J. N., Chang, F. H., Nodder, S. D., Northcote, L. C., Safi, K. A., Marriner, A., Langlois, R. J., LaRoche, J., Amosa, P., van Kooten, M., Feng, Y.-Y., Rowden, A. A., and Summerfield, T. C.: Predicting changes in plankton biodiversity & productivity of the EEZ in response to climate change induced ocean acidification, Ministry for Primary Industrie, Wellington, New Zealand, 200 pp., 2014.
Law, C. S., Bell, J. J., Bostock, H. C., Cornwall, C. E., Cummings, V. J., Currie, K., Davy, S. K., Gammon, M., Hepburn, C. D., Hurd, C. L., Lamare, M., Mikaloff-Fletcher, S. E., Nelson, W. A., Parsons, D. M., Ragg, N. L. C., Sewell, M. A., Smith, A. M., and Tracey, D. M.: Ocean acidification in New Zealand waters: trends and impacts, New Zeal. J. Mar. Fresh., 52, 155–195, https://doi.org/10.1080/00288330.2017.1374983, 2018.
Lawerence, C. and Menden-Deuer, S.: Drivers of protistan grazing pressure: seasonal signals of plankton community composition and environmental conditions, Mar. Ecol.-Prog. Ser., 459, 39–52, 2012.
Le Quéré, C., Harrison, S. P., Colin Prentice, I., Buitenhuis, E. T., Aumont, O., Bopp, L., Claustre, H., Cotrim Da Cunha, L., Geider, R., Giraud, X., Klaas, C., Kohfeld, K. E., Legendre, L., Manizza, M., Platt, T., Rivkin, R. B., Sathyendranath, S., Uitz, J., Watson, A. J., and Wolf-Gladrow, D.: Ecosystem dynamics based on plankton functional types for global ocean biogeochemistry models, Glob. Change Biol., 11, 2016–2040, https://doi.org/10.1111/j.1365-2486.2005.1004.x, 2005.
Le Quéré, C., Rödenbeck, C., Buitenhuis, E. T., Conway, T. J., Langenfelds, R., Gomez, A., Labuschagne, C., Ramonet, M., Nakazawa, T., Metzl, N., Gillett, N., and Heimann, M.: Saturation of the Southern Ocean CO2 sink due to recent climate change, Science, 316, 1735–1738, https://doi.org/10.1126/science.1136188, 2007.
Malinverno, E., Triantaphyllou, M. V., and Dimiza, M. D.: Coccolithophore assemblage distribution along a temperate to polar gradient in theWest Pacific sector of the Southern Ocean (January 2005), Micropaleontology, 61, 489–506, 2015.
Mayers, K. M. J., Poulton, A. J., Daniels, C. J., Wells, S. R., Woodward, E. M. S., Tarran, G. A., Widdicombe, C. E., Mayor, D. J., Atkinson, A., and Giering, S. L. C.: Growth and mortality of coccolithophores during spring in a temperate Shelf Sea (Celtic Sea, April 2015), Prog. Oceanogr., 177, 101928, https://doi.org/10.1016/j.pocean.2018.02.024, 2019.
McIntyre, A. and Bé, A. W. H.: Modern coccolithophoridae of the atlantic ocean – I. Placoliths and cyrtoliths, Deep Sea Research and Oceanographic Abstracts, 14, 561–597, https://doi.org/10.1016/0011-7471(67)90065-4, 1967.
McNeil, B. I. and Matear, R. J.: Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2, P. Natl. Acad. Sci. USA, 105, 18860–18864, 2008.
Medlin, L. K., Lange, M., and Baumann, M. E. M.: Genetic differentiation among three colony-forming species of Phaeocystis: further evidence for the phylogeny of the Prymnesiophyta, Phycologia, 33, 199–212, https://doi.org/10.2216/i0031-8884-33-3-199.1, 1994.
Metzl, N., Tilbrook, B., and Poisson, A.: The annual fCO2 cycle and the air–sea CO2 flux in the sub-Antarctic Ocean, Tellus B, 51, 849–861, https://doi.org/10.1034/j.1600-0889.1999.t01-3-00008.x, 1999.
Meyer, J. and Riebesell, U.: Reviews and Syntheses: Responses of coccolithophores to ocean acidification: a meta-analysis, Biogeosciences, 12, 1671–1682, https://doi.org/10.5194/bg-12-1671-2015, 2015.
Moy, A. D., Howard, W. R., Bray, S. G., and Trull, T. W.: Reduced calcification in modern Southern Ocean planktonic foraminifera, Nat. Geosci., 2, 276–280, 2009.
Müller, M. N., Trull, T. W., and Hallegraeff, G. M.: Differing responses of three Southern Ocean Emiliania huxleyi ecotypes to changing seawater carbonate chemistry, Mar. Ecol.-Prog. Ser., 531, 81–90, 2015.
Neukermans, G., Oziel, L., and Babin, M.: Increased intrusion of warming Atlantic water leads to rapid expansion of temperate phytoplankton in the Arctic, Glob. Change Biol., 24, 2545–2553, https://doi.org/10.1111/gcb.14075, 2018.
Nodder, S. D., Chiswell, S. M., and Northcote, L. C.: Annual cycles of deep-ocean biogeochemical export fluxes in subtropical and subantarctic waters, southwest Pacific Ocean, J. Geophys. Res.-Oceans, 121, 2405–2424, https://doi.org/10.1002/2015JC011243, 2016.
Northcote, L. C. and Neil, H. L.: Seasonal variations in foraminiferal flux in the Southern Ocean, Campbell Plateau, New Zealand, Mar. Micropaleontol., 56, 122–137, 2005.
O'Dea, S. A., Gibbs, S. J., Bown, P. R., Young, J. R., Poulton, A. J., Newsam, C., and Wilson, P. A.: Coccolithophore calcification response to past ocean acidification and climate change, Nat. Commun., 5, 5363, https://doi.org/10.1038/ncomms6363, 2014.
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., Gnanadesikan, A., Gruber, N., Ishida, A., and Joos, F.: Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms, Nature, 437, 681–686, 2005.
Orsi, A. H., Whitworth Iii, T., and Nowlin Jr., W. D.: On the meridional extent and fronts of the Antarctic Circumpolar Current, Deep-Sea Res. Pt. I, 42, 641–673, https://doi.org/10.1016/0967-0637(95)00021-W, 1995.
Paasche, E.: Coccolith Formation, Nature, 193, 1094–1095, https://doi.org/10.1038/1931094b0, 1962.
Paasche, E.: A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions, Phycologia, 40, 503–529, https://doi.org/10.2216/i0031-8884-40-6-503.1, 2002.
Pachauri, R. K., Allen, M. R., Barros, V. R., Broome, J., Cramer, W., Christ, R., Church, J. A., Clarke, L., Dahe, Q., and Dasgupta, P.: Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change, IPCC, Switzerland , p. 138, 2014.
Passow, U. and De La Rocha, C. L.: Accumulation of mineral ballast on organic aggregates, Global Biogeochem. Cy., 20, GB1013, https://doi.org/10.1029/2005GB002579, 2006.
Patarnello, T., Bargelloni, L., Varotto, V., and Battaglia, B.: Krill evolution and the Antarctic ocean currents: evidence of vicariant speciation as inferred by molecular data, Mar. Biol., 126, 603–608, https://doi.org/10.1007/bf00351327, 1996.
Patil, S. M., Mohan, R., Shetye, S. S., Gazi, S., Baumann, K.-H., and Jafar, S.: Biogeographic distribution of extant Coccolithophores in the Indian sector of the Southern Ocean, Mar. Micropaleontol., 137, 16–30, https://doi.org/10.1016/j.marmicro.2017.08.002, 2017.
Poulton, A. J., Charalampopoulou, A., Young, J. R., Tarran, G. A., Lucas, M. I., and Quartlya, G. D.: Coccolithophore dynamics in non-bloom conditions during late summer in the central Iceland Basin (July–August 2007), Limnol. Oceanogr., 55, 1601–1613, https://doi.org/10.4319/lo.2010.55.4.1601, 2010.
Poulton, A. J., Young, J. R., Bates, N. R., and Balch, W. M.: Biometry of detached Emiliania huxleyi coccoliths along the Patagonian Shelf, Mar. Ecol.-Prog. Ser., 443, 1–17, 2011.
Poulton, A. J., Painter, S. C., Young, J. R., Bates, N. R., Bowler, B., Drapeau, D., Lyczsckowski, E., and Balch, W. M.: The 2008 Emiliania huxleyi bloom along the Patagonian Shelf: Ecology, biogeochemistry, and cellular calcification, Global Biogeochem. Cy., 27, 1023–1033, https://doi.org/10.1002/2013gb004641, 2013.
Probert, I. and Houdan, A.: The laboratory culture of coccolithophores, in: Coccolithophores, Springer, Berlin, 217–249, 2004.
Quéguiner, B.: Iron fertilization and the structure of planktonic communities in high nutrient regions of the Southern Ocean, Deep-Sea Res. Pt. II, 90, 43–54, https://doi.org/10.1016/j.dsr2.2012.07.024, 2013.
Rigual-Hernández, A. S., Trull, T. W., Bray, S. G., Closset, I., and Armand, L. K.: Seasonal dynamics in diatom and particulate export fluxes to the deep sea in the Australian sector of the southern Antarctic Zone, J. Marine Syst., 142, 62–74, https://doi.org/10.1016/j.jmarsys.2014.10.002, 2015a.
Rigual-Hernández, A. S., Trull, T. W., Bray, S. G., Cortina, A., and Armand, L. K.: Latitudinal and temporal distributions of diatom populations in the pelagic waters of the Subantarctic and Polar Frontal zones of the Southern Ocean and their role in the biological pump, Biogeosciences, 12, 5309–5337, https://doi.org/10.5194/bg-12-5309-2015, 2015b.
Rigual Hernández, A. S., Flores, J. A., Sierro, F. J., Fuertes, M. A., Cros, L., and Trull, T. W.: Coccolithophore populations and their contribution to carbonate export during an annual cycle in the Australian sector of the Antarctic zone, Biogeosciences, 15, 1843–1862, https://doi.org/10.5194/bg-15-1843-2018, 2018.
Rigual-Hernández, A. S., Trull, T. W., and Nodder, S.: Coccolithophore species fluxes in the Australian and New Zealand sectors of the Subantarctic Zone, Australian Antarctic Data Centre, https://doi.org/10.26179/5ddf3db06a153, 2019.
Rigual-Hernández, A. S., Trull, T. W., Flores, J. A., Nodder, S. D., Eriksen, R., Davies, D. M., Hallegraeff, G. M., Sierro, F. J., Patil, S., Cortina, A., Ballegeer, A. M., Northcote, L. C., Abrantes, F., and Rufino, M. M.: Full annual monitoring of Subantarctic Emiliania huxleyi populations reveals highly calcified morphotypes in high-CO2 winter conditions, Sci. Rep.-UK, in review, 2020.
Rintoul, S. R. and Trull, T. W.: Seasonal evolution of the mixed layer in the Subantarctic zone south of Australia, J. Geophys. Res.-Oceans, 106, 31447–31462, https://doi.org/10.1029/2000JC000329, 2001.
Rintoul, S. R., Sparrow, M., Meredith, M. P., Wadley, V., Speer, K., Hofmann, E., Summerhayes, C., Urban, E., Bellerby, R., and Ackley, S.: The Southern Ocean observing system: initial science and implementation strategy, Scientific Committee on Antarctic Research, Cambridge, UK, 2012.
Rivero-Calle, S., Gnanadesikan, A., Del Castillo, C. E., Balch, W. M., and Guikema, S. D.: Multidecadal increase in North Atlantic coccolithophores and the potential role of rising CO2, Science, 350, 1533–1537, https://doi.org/10.1126/science.aaa8026, 2015.
Rost, B. and Riebesell, U.: Coccolithophores and the biological pump: responses to environmental changes, in: Coccolithophores: From Molecular Processes to Global Impact, edited by: Thierstein, H. R. and Young, J. R., Springer Berlin Heidelberg, Berlin, Heidelberg, 99–125, 2004.
Rousseaux, C. S. and Gregg, W. W.: Recent decadal trends in global phytoplankton composition, Global Biogeochem. Cy., 29, 1674–1688, 2015.
Saavedra-Pellitero, M. and Baumann, K.-H.: Comparison of living and surface sediment coccolithophore assemblages in the Pacific sector of the Southern Ocean, Micropaleontology, 61, 507–520, 2015.
Saavedra-Pellitero, M., Baumann, K.-H., Flores, J.-A., and Gersonde, R.: Biogeographic distribution of living coccolithophores in the Pacific sector of the Southern Ocean, Mar. Micropaleontol., 109, 1–20, 2014.
Sabine, C. L., Feely, R. A., Gruber, N., Key, R. M., Lee, K., Bullister, J. L., Wanninkhof, R., Wong, C. S., Wallace, D. W. R., Tilbrook, B., Millero, F. J., Peng, T.-H., Kozyr, A., Ono, T., and Rios, A. F.: The Oceanic Sink for Anthropogenic CO2, Science, 305, 367–371, https://doi.org/10.1126/science.1097403, 2004.
Salter, I., Schiebel, R., Ziveri, P., Movellan, A., Lampitt, R., and Wolff, G. A.: Carbonate counter pump stimulated by natural iron fertilization in the Polar Frontal Zone, Nat. Geosci., 7, 885–889, https://doi.org/10.1038/ngeo2285, 2014.
Samtleben, C. and Bickert, T.: Coccoliths in sediment traps from the Norwegian Sea, Mar. Micropaleontol., 16, 39–64, https://doi.org/10.1016/0377-8398(90)90028-K, 1990.
Schiebel, R. and Hemleben, C.: Planktic foraminifers in the modern ocean, Springer, Berlin, 2017.
Schiebel, R., Spielhagen, R. F., Garnier, J., Hagemann, J., Howa, H., Jentzen, A., Martínez-Garcia, A., Meilland, J., Michel, E., Repschläger, J., Salter, I., Yamasaki, M., and Haug, G.: Modern planktic foraminifers in the high-latitude ocean, Mar. Micropaleontol., 136, 1–13, https://doi.org/10.1016/j.marmicro.2017.08.004, 2017.
Schlitzer, R.: Ocean Data View, available at: https://odv.awi.de (last access: 15 January 2020), 2018.
Shadwick, E. H., Trull, T. W., Thomas, H., and Gibson, J. A. E.: Vulnerability of Polar Oceans to Anthropogenic Acidification: Comparison of Arctic and Antarctic Seasonal Cycles, Sci. Rep.-UK, 3, 2339, https://doi.org/10.1038/srep02339, 2013.
Sinha, B., Buitenhuis, E. T., Quéré, C. L., and Anderson, T. R.: Comparison of the emergent behavior of a complex ecosystem model in two ocean general circulation models, Prog. Oceanogr., 84, 204–224, https://doi.org/10.1016/j.pocean.2009.10.003, 2010.
Sloyan, B. M. and Rintoul, S. R.: Circulation, Renewal, and Modification of Antarctic Mode and Intermediate Water, J. Phys. Oceanogr., 31, 1005–1030, https://doi.org/10.1175/1520-0485(2001)031<1005:cramoa>2.0.co;2, 2001a.
Sloyan, B. M. and Rintoul, S. R.: The Southern Ocean Limb of the Global Deep Overturning Circulation, J. Phys. Oceanogr., 31, 143–173, https://doi.org/10.1175/1520-0485(2001)031<0143:TSOLOT>2.0.CO;2, 2001b.
Sokolov, S. and Rintoul, S. R.: Circumpolar structure and distribution of the Antarctic Circumpolar Current fronts: 2. Variability and relationship to sea surface height, J. Geophys. Res.-Oceans, 114, C11019, https://doi.org/10.1029/2008JC005248, 2009.
Thornhill, D. J., Mahon, A. R., Norenburg, J. L., and Halanych, K. M.: Open-ocean barriers to dispersal: a test case with the Antarctic Polar Front and the ribbon worm Parborlasia corrugatus (Nemertea: Lineidae), Mol. Ecol., 17, 5104–5117, https://doi.org/10.1111/j.1365-294X.2008.03970.x, 2008.
Trull, T. W., Bray, S. G., Manganini, S. J., Honjo, S., and François, R.: Moored sediment trap measurements of carbon export in the Subantarctic and Polar Frontal zones of the Southern Ocean, south of Australia, J. Geophys. Res.-Oceans, 106, 31489–31509, https://doi.org/10.1029/2000JC000308, 2001.
Trull, T. W., Schulz, E., Bray, S. G., Pender, L., McLaughlan, D., Tilbrook, B., Rosenberg, M., and Lynch, T.: The Australian Integrated Marine Observing System Southern Ocean Time Series facility, OCEANS 2010 IEEE – Sydney, https://doi.org/10.1109/OCEANSSYD.2010.5603514, 1–7, 2010.
Trull, T. W., Passmore, A., Davies, D. M., Smit, T., Berry, K., and Tilbrook, B.: Distribution of planktonic biogenic carbonate organisms in the Southern Ocean south of Australia: a baseline for ocean acidification impact assessment, Biogeosciences, 15, 31–49, https://doi.org/10.5194/bg-15-31-2018, 2018.
Tyrrell, T. and Merico, A.: Emiliania huxleyi: bloom observations and the conditions that induce them, in: Coccolithophores, Springer, Berlin, 75–97, 2004.
Volk, T. and Hoffert, M. I.: Ocean Carbon Pumps: Analysis of Relative Strengths and Efficiencies in Ocean-Driven Atmospheric CO2 Changes, in: The Carbon Cycle and Atmospheric CO2: Natural Variations Archean to Present, American Geophysical Union, Washington, DC, 99–110, 1985.
Winter, A., Henderiks, J., Beaufort, L., Rickaby, R. E., and Brown, C. W.: Poleward expansion of the coccolithophore Emiliania huxleyi, J. Plankton Res., 36, 316–325, 2014.
Young, J., Geisen, M., Cross, L., Kleijne, A., Sprengel, C., Probert, I., and Østergaard, J.: A guide to extant coccolithophore taxonomy, Journal of Nanoplankton Research, Special Issue 1, International Nannoplankton Association, 2003.
Young, J. R.: The description and analysis of coccolith structure, Nannoplankton Research, in: Coccolithophores, edited by: Hamrsmid, B. and Young, J. R., ZPZ, Knihovnicha, 35–71, 1992.
Young, J. R. and Ziveri, P.: Calculation of coccolith volume and it use in calibration of carbonate flux estimates, Deep-Sea Res. Pt. II, 47, 1679–1700, https://doi.org/10.1016/S0967-0645(00)00003-5, 2000.
Young, J. R., Davis, S. A., Bown, P. R., and Mann, S.: Coccolith Ultrastructure and Biomineralisation, J. Struct. Biol., 126, 195–215, https://doi.org/10.1006/jsbi.1999.4132, 1999.
Young, J. R., Bown, P. R., and Lees, J. A.: Nannotax3 website, International Nannoplankton Association, available at: http://www.mikrotax.org/Nannotax3, last access: July 2019.
Zhang, X., Lewis, M., Lee, M., Johnson, B., and Korotaev, G.: The volume scattering function of natural bubble populations, Limnol. Oceanogr., 47, 1273–1282, 2002.
Ziveri, P., Broerse, A. T. C., van Hinte, J. E., Westbroek, P., and Honjo, S.: The fate of coccoliths at 48∘ N 21∘ W, Northeastern Atlantic, Deep-Sea Res. Pt. II, 47, 1853–1875, https://doi.org/10.1016/S0967-0645(00)00009-6, 2000.
Ziveri, P., de Bernardi, B., Baumann, K.-H., Stoll, H. M., and Mortyn, P. G.: Sinking of coccolith carbonate and potential contribution to organic carbon ballasting in the deep ocean, Deep-Sea Res. Pt. II, 54, 659–675, https://doi.org/10.1016/j.dsr2.2007.01.006, 2007.