the Creative Commons Attribution 3.0 License.
the Creative Commons Attribution 3.0 License.
Carbon mineralization in Laptev and East Siberian sea shelf and slope sediment
Volker Brüchert
Lisa Bröder
Joanna E. Sawicka
Tommaso Tesi
Samantha P. Joye
Xiaole Sun
Igor P. Semiletov
Vladimir A. Samarkin
The Siberian Arctic Sea shelf and slope is a key region for the degradation of terrestrial organic material transported from the organic-carbon-rich permafrost regions of Siberia. We report on sediment carbon mineralization rates based on O2 microelectrode profiling; intact sediment core incubations; 35S-sulfate tracer experiments; pore-water dissolved inorganic carbon (DIC); δ13CDIC; and iron, manganese, and ammonium concentrations from 20 shelf and slope stations. This data set provides a spatial overview of sediment carbon mineralization rates and pathways over large parts of the outer Laptev and East Siberian Arctic shelf and slope and allows us to assess degradation rates and efficiency of carbon burial in these sediments. Rates of oxygen uptake and iron and manganese reduction were comparable to temperate shelf and slope environments, but bacterial sulfate reduction rates were comparatively low. In the topmost 50 cm of sediment, aerobic carbon mineralization dominated degradation and comprised on average 84 % of the depth-integrated carbon mineralization. Oxygen uptake rates and anaerobic carbon mineralization rates were higher in the eastern East Siberian Sea shelf compared to the Laptev Sea shelf. DIC ∕ NH ratios in pore waters and the stable carbon isotope composition of remineralized DIC indicated that the degraded organic matter on the Siberian shelf and slope was a mixture of marine and terrestrial organic matter. Based on dual end-member calculations, the terrestrial organic carbon contribution varied between 32 and 36 %, with a higher contribution in the Laptev Sea than in the East Siberian Sea. Extrapolation of the measured degradation rates using isotope end-member apportionment over the outer shelf of the Laptev and East Siberian seas suggests that about 16 Tg C yr−1 is respired in the outer shelf seafloor sediment. Of the organic matter buried below the oxygen penetration depth, between 0.6 and 1.3 Tg C yr−1 is degraded by anaerobic processes, with a terrestrial organic carbon contribution ranging between 0.3 and 0.5 Tg yr−1.
- Article
(8250 KB) -
Supplement
(386 KB) - BibTeX
- EndNote
The biogeochemical fate of terrestrial organic carbon deposited on the Arctic shelf and slope is one of the most important open questions for the marine Arctic carbon cycle (e.g., Tesi et al., 2014; Macdonald et al., 2015; McGuire et al., 2009; Vonk et al., 2012). The total pan-Arctic terrestrial permafrost region has been estimated to contain about 1100–1500 Pg of carbon, of which 500 Pg is seasonally or perennially unfrozen and contributes to the present-day carbon cycle (Hugelius et al., 2014). Additional partial thawing, mobilization, and oxidation of the perennially frozen carbon reservoir can substantially affect the global atmospheric carbon dioxide pool over the next 100 years (Schuur et al., 2015; Koven et al., 2015). A key problem in this context is the considerable uncertainty regarding the mineralization of terrestrial organic matter exported by rivers and coastal erosion to the Siberian shelf and slope (Tesi et al., 2014; Karlsson et al. 2015; Semiletov et al., 2011; Salvado et al., 2015).
Terrestrial organic matter transported to the Siberian shelf is of variable size, age, and molecular composition, which results in a range of different carbon degradation rates of bulk carbon and individual molecular components. Size class analysis of the organic matter suggests that coarse organic material settles preferentially in near-shore environments, whereas finer organic fractions disperse offshore in repeated deposition–resuspension cycles, gradually losing particular molecular components and overall reactivity (Wegner et al., 2013; Tesi et al., 2014, 2016). Substantial oxic degradation of organic matter may occur during near-bottom transport in resuspension–deposition cycles across the shelf (Bröder et al., 2016a). Up to 90 % of certain biomarker classes may decompose during transport, whereby most of the degradation may take place while the transported organic material resides in the sediment before being resuspended (Bröder et al., 2016a). However, without making approximations on transport direction, particle travel time, and travel distance, these studies cannot provide direct insights into the rates of carbon degradation and resultant CO2 fluxes from sediment. By contrast, direct kinetic constraints provided by sediment carbon degradation rates can provide testable data for coupled hydrodynamic biogeochemical models that help assess the fate of the land-exported terrestrial carbon pool on the Siberian shelf.
Relatively few studies have directly measured rates of carbon mineralization in Siberian shelf sediment (e.g., Boetius and Damm, 1998; Grebmeier et al., 2006; Karlsson et al., 2015; Savvichev et al., 2007). Boetius and Damm (1998) used high-resolution oxygen microelectrode data to determine the surface oxygen concentration gradients and oxygen penetration depths in a large number of sediment cores from the shelf and slope of the Laptev Sea. Based on corresponding sediment trap and export productivity data, they concluded that the annual marine organic carbon export in the Laptev Sea shelf and slope was sufficiently high to explain the observed oxygen uptake rates. Current understanding therefore holds that due to the long annual ice cover and low productivity on the eastern Siberian Arctic shelf and slope, only a small amount of marine organic carbon is exported and buried in Laptev and East Siberian sea shelf sediment. The highly reactive fraction of fresh organic matter is thought to degrade in the surface sediment (Boetius and Damm, 1998). Consequently, anaerobic respiration in buried sediment has been thought to be negligible and to reflect the degradation of unreactive terrestrially derived carbon compounds. To our knowledge, with the exception of a recent study by Karlsson et al. (2015), a more direct assessment of terrestrial carbon-derived mineralization rates in buried shelf and slope sediment has not been reported for the East Siberian Arctic Sea.
In this study, we present data from oxygen microelectrode profiling experiments, pore-water data of dissolved inorganic carbon (DIC) and its stable carbon isotope composition, and 35S-sulfate reduction rate experiments along a shelf–slope transect near 125∘ E in the Laptev Sea. Samples were taken during summer 2014 on the SWERUS-C3 expedition with the Swedish icebreaker Oden. We combined these data with pore-water analyses of dissolved ammonium, sulfate, iron, and manganese to assess the major carbon degradation pathways and rates across the extensive outer Laptev and Siberian shelf and slope.
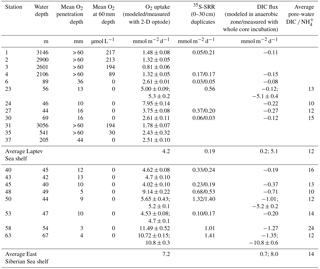
Table 1Physical and chemical characteristics of sediment and bottom water at the sampled stations; n.a.: not analyzed.
2.1 Sample collection
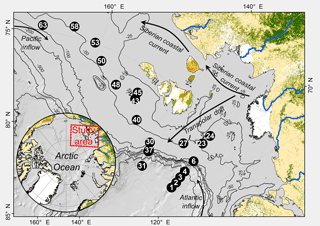
Samples were collected at 20 stations from 40 to 3146 m water depth in the western Laptev and East Siberian seas (Fig. 1 and Table 1). In this study we only report on sampling sites that showed no methane gas plumes, acoustic anomalies in the water column, or sediment blankings indicative of rising gas. In areas of active ebullition from the seafloor as seen by video imagery and acoustic gas blankings in the water column, the biogeochemistry of seafloor processes such as bacterial sulfate reduction, DIC concentration and its carbon isotope composition, and oxygen uptake are affected by methane oxidation. These methane-cycling-related signals overprint the biogeochemistry imparted by carbon mineralization and are reported in a separate study.
Sediment stations had variable ice cover (Table 1). In the Laptev Sea, except for the deep-water slope stations between 3146 and 2106 m, all stations had open water. By contrast, ice cover exceeded 75 % in the East Siberian Sea to the west and east of Bennett Island (Stations 40 to 63). Sediments with well-preserved sediment surfaces were collected with a multicorer (Oktopus GmbH, Kiel, Germany) that simultaneously takes eight sediment cores over an area of about 0.36 m2 with acrylic tubes (9.5 cm diameter, 60 cm length) to 40 cm depth, preserving clear water on top of the sediment. At Stations 6, 23, and 24, an underwater video system (Group B Distribution Inc., Jensen Beach, USA) was mounted on the multicore frame to record the deployment and recovery and to document the seafloor habitat. For the analyses all cores were taken from the same cast. Two of the cores were used to determine 35S-sulfate reduction rates and porosity. In addition, one core with predrilled 3.8 mm holes sealed with electric tape was used to extract pore waters with rhizons (Rhizosphere Research Products BV, Wageningen, Netherlands). A fourth core was used for microelectrode measurements of dissolved oxygen concentration profiling, and finally, four other cores were used for whole-core incubations to determine benthic fluxes of dissolved oxygen, DIC, and nutrients. The cores were capped with rubber stoppers until further subsampling, usually within 30 min. For sulfate reduction rates, the cores were subsampled with 40 or 50 cm long acrylic tubes (26 mm inner diameter) prepared with silicon-sealed holes, drilled at distances of 1 cm. For whole-core incubations, the cores were subsampled with 25 cm long, 60 mm wide tubes (56 mm inner diameter, id) to 12 cm depth. Likewise, a 60 mm diameter tube (56 mm id) was collected for microelectrode measurements preserving about 3 cm of the overlying bottom water. For intact whole-core incubations, temperature-controlled aquaria were filled with bottom water that was collected from a CTD rosette from the same station by collecting water from four 10 L rosette bottles usually ∼ 5 m above the seafloor. All sediment cores were closed with a stopper, retaining the water on top of the sediment and stored at 1.5 ∘C in an incubator until further processing.
2.2 Microelectrode oxygen profiles
High-resolution O2 profiles across the water–sediment interface were obtained to determine oxygen penetration depths and diffusive oxygen uptake (Rasmussen and Jørgensen, 1992; Glud, 2008; Table 2). The 60 mm tubes were placed in an aquarium filled with bottom water from the same station, overflowing the sediment core. The water temperature was kept to ∼ 1 ∘C by a cooling unit (Julabo GmbH, Seelbach, Germany). In exceptional cases in which there was not sufficient bottom water available to fill the aquarium, bottom water was used from a pump system. A stable diffusive boundary layer above the sediment was created by passing air from an aquarium pump over the water surface with a Pasteur pipette, creating a slow rotational motion of water inside the core. At each station six to eight O2 microprofiles were measured using Clark-type oxygen microelectrodes (OX-50, Unisense, Aarhus, Denmark) mounted on a motor-driven micromanipulator (MM33, Unisense, Aarhus, Denmark). O2 sensors were calibrated with fully oxygenated bottom water from the same station at ∼ 1 ∘C for saturation and for anoxic conditions by dissolving Na2SO3 in the same water. The first profile in each core was measured with a resolution of 1000 µm as a quick scan to locate the sediment surface and to adjust the measuring range. Then the vertical resolution was increased to 100–500 µm and an additional five to seven profiles were measured at different points on the surface, approximately 1 cm apart from each other.
2.3 Whole-core sediment incubations
Four intact cores with clear overlying water were subsampled in the laboratory in acrylic tubes (56 mm id, height 25 cm) retaining about 10 cm of the overlying water. The sediment and water height in the tubes was approximately 10 cm. The cores were incubated in a 40 L incubation tank filled with bottom water from the same station. Before the incubation the overlying water in the cores was equilibrated with bottom water in the tank. The overlying water in the cores was stirred by small magnetic bars mounted in the core liners and driven by an external magnet at 60 rpm. The cores were preincubated uncapped for 6 h and subsequently capped and incubated for a period of 6 to 24 h depending on the initial oxygen concentration in the bottom water. Two-dimensional oxygen sensor spots (Firesting oxygen optode, Pyro Science GmbH, Aachen, Germany) with a sensing surface of a diameter of 5 mm were attached to the inner wall of two incubation cores. The sensor spots were calibrated against O2-saturated bottom water and oxygen-free water following the manufacturer's guidelines, accounting for temperature and salinity of the incubation water. Measurements were performed with a fiber-optic cable connected to the spot adapter fixed at the outer core liner wall at the spot position. The O2 concentration was continuously logged during incubations. Sediment total oxygen uptake rates were computed using linear regression of the O2 concentration over time. An amount of 5 mL of overlying water was removed over the course of the incubation used for DIC analysis as described below. Linear regression best fits were used to determine the exchange fluxes of DIC.
2.4 Extracted pore-water analysis
Pore-water samples for concentration measurements of DIC, sulfate (SO, and ammonium (NH were obtained using the methods described in Seeberg-Elverfeldt et al. (2005). Rhizons were treated for 2 h in 10 % HCl solution, followed by two rinses with deionized water for 2 h and final storage in deionized water. The rhizons were connected to 10 mL disposable plastic syringes with inert pistons (VWR International, Stockholm, Sweden) via polyethylene three-way luer-type stopcocks (Cole-Parmer, USA) and inserted in 1 cm intervals through tight-fitting, predrilled holes in the liner of the sediment cores. The first milliliter of pore water was discarded from the syringe. No more than 5 mL was collected from each core to prevent cross-contamination of adjacent pore water due to the suction effect (Seeberg-Elverfeldt et al., 2005). The collected pore water was divided into four different aliquots for later chemical analysis. For dissolved sulfate analysis, 1 mL of pore water was preserved with 200 µL of 5 % zinc acetate solution and frozen. For inductively coupled plasma atomic emission spectrometry (ICP-AES) analysis of dissolved metals and major cations, 1 mL of pore water was preserved with 100 µL of 10 % Suprapur HNO3 and stored cold. For analysis of dissolved ammonium, 2 mL of pore water was frozen untreated. For analysis of DIC, 2 mL of pore water was preserved with 100 µL of 10 % HgCl2 and stored cold in brown glass vials without headspace. Ammonium was determined on a QuAAtro four-channel flow injection analyzer (Seal Analytical) onboard. All other pore-water analyses were performed at the Department of Geological Sciences, Stockholm University. Samples that were analyzed in the home laboratory remained cold or frozen onboard until arrival of the icebreaker Oden in Sweden. Sulfate concentration was measured on diluted aliquots on a Dionex system IC 20 ion chromatograph. DIC concentrations were determined with flow injection analysis (Hall and Aller, 1992). Dissolved iron and manganese were determined on diluted aliquots using ICP-AES (Varian Vista AX). For carbon isotope analysis of DIC, 1 mL of pore water was filled into 12 mL Exetainers to which 1 mL of concentrated phosphoric acid was added. The carbon isotope composition of the formed CO2 was analyzed on a GasBench II-MAT 253 isotope ratio mass spectrometer coupled to a gas chromatography PAL autosampler. Results are reported in the conventional delta notation relative to VPDB. Precision of isotope analysis is 0.1 ‰.
For the calculation of pore-water concentration ratios of DIC and NH, the effects of different diffusion coefficients and ammonium adsorption were accounted for. We have no direct measurements of adsorption coefficients for these sediments. Instead, we used an ammonium adsorption coefficient of 1.3 established for comparable, terrestrially dominated silty clays in the East China Sea for which similar porosities and organic carbon concentrations were reported (Mackin and Aller, 1984). The diffusion coefficient of HCO is about 45 % smaller than the diffusion coefficient of NH (Li and Gregory, 1974). The two effects required an upward correction of the ammonium concentration by 40 % to facilitate direct comparison in DIC ∕ NH ratios. Diffusion- and adsorption-adjusted DIC ∕ NH ratios were also corrected for the bottom-water DIC and NH concentrations (Table 1). Only concentrations below 4 cm depth were used for comparison to avoid effects of oxidation on NH concentrations.
2.5 Reaction transport modeling
Reaction rates and fluxes were estimated from the concentration profiles of dissolved oxygen, manganese, iron, and DIC according to the general reaction–transport equation at steady state accounting for diffusion and advection described here for dissolved oxygen according to
At steady state, the rate of the concentration change reflects the balance between the consumption due to respiration and oxidation of reduced inorganic compounds (R) against diffusion and advection due to bioirrigation into sediment (Glud, 2008). Ds (cm2 s−1) is the sediment diffusion coefficient and was calculated for the experimental temperature and salinity according to Boudreau (1996). The sediment diffusion coefficient Ds was recalculated from the molecular diffusion coefficient Do according to , where , where ϕ is porosity and θ is tortuosity (Boudreau, 1997). Db (cm2 s−1) is the bioturbation coefficient and α is the irrigation coefficient (cm s−1). Db and α were estimated with stepwise optimization by fitting a concentration profile to the measured data using the least-square fitting procedure of the program Profile (Berg et al., 1998) testing various coefficients until the statistically best fit was obtained. Boundary conditions and the coefficients Db and α for the best fits are shown in the Supplement Table S1.
2.6 35S-Sulfate reduction rates (35SRR)
For the incubations, the whole-core incubation method from Jørgensen (1978) was used. 35SO tracer solution was diluted in a 6 ‰ NaCl solution containing 0.5 mM SO, and 2.5 µL of the tracer solution (200 kBq) was injected through the predrilled holes. The cores were then capped and sealed in plastic wrap foil and incubated for 8 h at the respective bottom-water temperatures. After this time, the incubations were stopped by sectioning the core in 1 cm intervals to 5 cm depth and in 2 cm intervals below this depth to the bottom of the core. Sediment sections were transferred into 50 mL plastic centrifuge tubes containing 20 mL zinc acetate (20 % v∕v), mixed to a slurry on a vortex stirrer, and frozen. The total amount of 35S-labeled reduced inorganic sulfur (TRIS) was determined using the single-step cold chromium distillation method from Kallmeyer et al. (2004). TRIS and supernatant sulfate were counted on a Tri-Carb 2095 Perkin Elmer scintillation counter. The sulfate reduction rate was calculated using the following equation (Jørgensen, 1978):
where [SO] is the pore-water sulfate concentration corrected for porosity ρ, TRI35S and 35SO are the measured counts (cpm) of sulfate and total reduced inorganic sulfur species, respectively, 1.045 is a correction factor accounting for the kinetic isotope effect of 35S relative to 32S, and T is the incubation time. The sulfate reduction rate is reported as nmol cm−3 day−1. 35SRR values were measured in two parallel cores for all depth intervals. The incubation experiments were conducted between 15 July and 20 August, but for logistical reasons (transport to Stockholm) the distillation of the samples was conducted between 10 December 2014 and 2 April 2015 so that between 1.7 and 2.7 half-lives of 35S (87.4 days) had passed before all samples were processed. The resulting detection limit of the rate measurements accounting for distillation blanks and radioactive decay of 35S between experiment and laboratory workup was 0.01 nmol cm−3 d−1.
2.7 Carbon equivalent apportionment of terminal electron-accepting processes
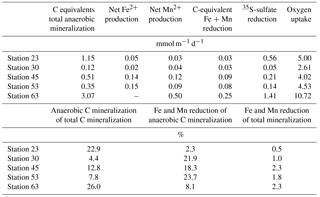
The modeled oxygen, iron, manganese, and DIC reaction rates were integrated over 30 cm sediment depth to permit comparison between different stations. The integrated terminal electron-accepting processes (TEAP) were recalculated into carbon equivalents for the electron acceptor oxygen, iron, manganese, and sulfate using an idealized (CH2O)x stoichiometry for organic matter (Vandieken et al., 2006; Nickel et al., 2008). The calculated rates were then used to calculate the contribution of the different aerobic and anaerobic electron acceptors to total carbon mineralization for five stations in the Laptev and East Siberian seas (Table 3).
2.8 Marine versus terrestrial end-member partitioning of carbon degradation rates
In order to determine the proportion of mineralized terrestrial and marine organic matter in pore-water DIC, we used a two-end-member isotope mass balance model. Pore-water DIC is assumed to be derived from bottom-water DIC and remineralization of organic matter during burial. We used a Keeling-type plot of 1/DIC against the corresponding stable carbon isotope composition of DIC to determine the isotope composition of the remineralized end-member by linear regression (Fig. 5b). The method assumes that diffusion is slow compared to burial and mineralization and that isotope fractionation during the oxidation of organic matter is negligible. Removal or addition of DIC by diagenetic processes such as CaCO3 precipitation or dissolution was minor. This is supported by the observation that the pore-water concentrations of Ca2+ and Mg2+ at these shelf and slope stations did not change significantly with depth (Brüchert and Sun, unpublished data). The relative contribution of the terrestrial and marine organic carbon was calculated with a linear two-end-member isotope model:
where fterr and fmar are the respective mass fractions of terrestrial and marine-derived organic carbon and δ13Cterr OC and δ13Cmarine OC reflect the isotope composition of these end-members. For the Laptev Sea shelf, a fraction of the surface water DIC used for marine production is derived from terrestrial dissolved organic carbon (DOC) and particulate organic carbon (POC) remineralized in shelf waters and transported from land (Tesi et al., 2017; Alling et al., 2012). δ13C values of offshore sediment in the Laptev Sea sediment are as heavy as −22.3 ‰ (Salvado et al., 2015). Tesi et al. (2017) report δ13C of POC of −24.7 ‰ for the outer Laptev Sea near the ice edge containing abundant dinoflagellates and only trace indicators of terrestrial organic carbon. Based on these data we use an isotope end-member composition of −23.0 ‰ for marine organic matter in the Laptev Sea. We are aware that there may be regional variations in this end-member composition. An isotope composition of −28 ‰ was used for the terrestrial organic carbon contribution in the Laptev Sea (Vonk et al., 2012). For the East Siberian Sea east of 140∘ E, the heaviest calculated isotope composition of remineralized pore-water DIC was −19 ‰ and is used here as the marine end-member (Station 53). The same carbon isotope composition of −28 ‰ as for the Laptev Sea was used as the terrestrial end-member. In order to derive specific degradation rates of the marine and terrestrial carbon fractions, the end-member mixing-based assessment of the marine and terrestrial organic carbon contributions to DIC was combined with the mineralization rates derived for the different electron acceptors.
3.1 Physical and chemical bottom-water conditions
Table 1 summarizes the general site characteristics of the investigated sediment stations. Bottom-water salinity varied between 34.9 ‰ in the outer Laptev Sea at 3146 m depth (Station 1) and 29.1 ‰ in the East Siberian Sea at 40 m (Station 45). The lower salinity in the East Siberian Sea can be attributed to longshore transport of freshwater eastward from the Lena River. Bottom-water temperatures varied between −1.8 ∘C at Station 27 and 0 ∘C at Station 37, but there was no regional trend in the data. Cored sediment consisted of silty clays to clayey silts. Slope sediment had a distinctly brown color throughout the cored interval, whereas shelf sediment only had a 1 to 4 cm thick brown interval, below which the sediment color changed to gray. In the eastern part of the East Siberian Sea, the sediment was mottled black below 10 cm depth. Iron–manganese concretions were found between 2 cm and 10 cm depth at Stations 24, 42, and 43 but were also observed at other stations on the shelf that were not part of this study. Benthic macrofauna, when present, consisted mainly of brittle stars, isopods, few polychaetes, and rare bivalves. All bottom waters were well-oxygenated with concentrations higher than 190 µmol L−1, but the shelf bottom waters in the East Siberian Sea had generally lower concentrations than in the Laptev Sea and bottom waters on the continental slope had lower oxygen concentrations than on the shelf. Concentrations of bottom-water ammonium ranged between 0.2 and 1.8 µmol L−1. Generally, the slope stations and the shelf stations closest to the Lena delta had the highest ammonium concentrations, whereas the other shelf stations showed no clear regional trend other than proximity to the Lena delta. Bottom-water DIC concentrations varied between 2086 (Station 53) and 2598 µM (Station 27), and the stable carbon isotope composition of DIC in the waters overlying the cores was between −0.5 and −6.5 ‰ vs. Vienna Pee Dee Belemnite (VPDB).
3.2 Dissolved oxygen, manganese, and iron
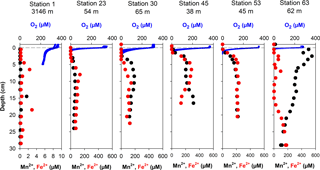
We show representative profiles of oxygen concentrations in Fig. 2 for the Laptev Sea slope station 1, the Laptev Sea shelf Stations 23, 30, and 45, and the East Siberian Sea shelf Stations 53 and 63. Oxygen penetration depths varied between 3 mm at Station 58 and more than 60 mm in all slope sediments (Table 2). For the Laptev Sea slope Stations 1, 2, 3, and 4, the maximum depth of oxygen penetration could not be determined because at penetration greater than 60 mm the conical sensor needle opened a hole in the sediment through which oxygen-containing bottom water could have potentially entered the sediment at depth thereby artificially extending the oxygen penetration depth. In the slope-to-shelf transects the oxygen penetration depth decreased from > 60 mm off-shore to 10 mm at the most inshore station in the Laptev Sea and the East Siberian Sea. At the two easternmost shelf Stations 58 and 63, we measured the lowest oxygen penetration depths: 3 and 4 mm, respectively. Evidence for bioturbation and bioirrigation based on multiple microelectrode profile measurements per core was rare. Only at Station 48 was a clear increase in oxygen concentrations below the sediment surface observed, indicative of active bioirrigating macrofauna. However, even at this station, based on investigations in parallel multicore casts, fauna was not abundant (A. Gukov, personal communication, 2014). At all other stations, oxygen concentrations decreased exponentially with depth. Fitting of the oxygen concentration profiles to the steady advection–diffusion–transport model (Eq. 1) yielded fluxes that varied between 0.81 and 11.49 mmol m−2 d−1 (Table 2). These calculated O2 fluxes compared well with total oxygen uptake rates calculated from whole-core incubations using 2-D optode sensor spots (Table 2). The good fit between the two methods also supports the notion that bioirrigation and bioturbation effects from meiofauna and macrofauna were minor.
In the slope sediment at Station 1, concentrations of dissolved manganese and iron were low throughout the cored depth interval and below 0.2 and 0.5 µM, respectively. The exception was a small increase for both elements between 4 and 8 cm depth and 14 and 20 cm depth to concentrations of less than 3 µM, possibly due to slightly more degradable organic material in these depth intervals (Fig. 2). A similar concentration profile was found for the other slope Station 4 (data not shown), but here concentrations were below 2 µM throughout for both dissolved iron and manganese and only slightly higher in the topmost centimeter. On the shelf, in the Laptev Sea (Station 23 and 30), concentrations of dissolved manganese and iron were below 0.3 and 1.5 µM, respectively, in the top 2 and 3 cm at Station 23, before increasing to maximum concentrations of 69 and 134 µM. At both stations, metal concentrations decreased again below the concentration maximum, indicating that deeper buried sediment was not a source of the metals and that the dominant source of iron and manganese was reduction in the topmost 5 cm of sediment. There was a general trend of increasing manganese concentrations from west to the east. At Station 30 in the Laptev Sea, the concentration of dissolved manganese was less than 0.3 µM in the topmost centimeter, but increased steeply to maximum concentrations of 189 µM at 9 cm depth. Similarly, dissolved iron concentrations were below 1 µM to 4 cm depth and then increased to 144 µM. Again, below the maximum, both iron and manganese pore-water concentrations decreased with increasing sediment depth. Even higher iron and manganese concentrations were found in the East Siberian Sea (Stations 45, 53, 63), where dissolved manganese already increased from the bottom water to concentrations of 20 µM in the topmost centimeter of sediment, and iron increased to above 1 µM below 2 cm depth. The steepest manganese concentration gradient was found at Station 63 in the easternmost East Siberian Sea, where concentrations were 501 µM in the first centimeter of sediment with a concentration maximum of 548 µM at 2.5 cm depth, decreasing below this depth to 115 µM at 30 cm depth. Station 63 differed with respect to dissolved iron concentrations from the other stations, because here dissolved iron showed two small peaks at 3.5 and 7 cm, and concentration increased substantially only below 17 cm depth to 189 µM.
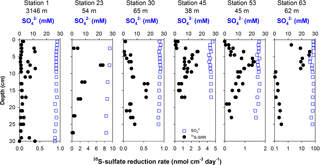
3.3 35S-sulfate reduction rates and pore-water sulfate
Sulfate concentrations showed minor depth gradients at all sampling sites (Fig. 3) and decreased from starting concentrations between 23.9 and 28.1 mM by 0.4 to 2.5 mM from the top to the bottom of the cores. At all stations, turnover of 35S tracer was recorded from the topmost sediment interval to the bottom of the core, indicating active bacterial sulfate reduction (Fig. 3). Depth-integrated rates over the recovered core lengths varied between 0.03 and 1.41 mmol m−2 d−1 (Table 2). The integrated rates were lowest at Station 1 at 3146 m in the Laptev Sea and highest at Station 63 in the easternmost East Siberian Sea. Across the shelf (Stations 6 to 24), depth-integrated rates increased from west to the east. Examples of sulfate reduction profiles are shown in Fig. 3 for the same six stations as previously. At Station 1, these rates ranged from 0.03 to 0.38 nmol cm−3 d−1. At this station, the variability between replicate cores was large, likely because many rates were near the detection limit in our handling procedure. Overall, sulfate reduction was higher in the top 10 cm of sediment but showed no pronounced change with depth at this station. This suggests that the reactivity of the organic material did not change substantially over the cored depth interval. The second slope station, Station 4, showed a similar rate–depth profile to Station 1. Depth profiles for the mid-outer shelf Stations 23 to 63 all showed broad subsurface maxima between 2.5 and 17.5 cm, but the depths of the rate maxima differed between the different stations (Fig. 3). Peak rates varied between 0.6 nmol cm−3 d−1 at Station 30 and 39 nmol cm−3 d−1 at Station 63. The second highest rate, 7.6 nmol cm−3 d−1, was found at the station closest to the Lena delta, Station 23. At all stations, sulfate reduction rates decreased from the maxima to rates below 1 nmol cm−3 d−1 or below the detection limit at the bottom of the cores. A particularly sharp decrease in the sulfate reduction rate was observed between 8 and 9 cm at Station 63, where rates dropped from 8.5 to 0.1 nmol cm−3 d−1 over 1 cm depth. Since sulfate was abundant throughout the cored intervals, this order-of-magnitude decrease indicates substantial changes in the reactivity of buried organic matter. Although no abrupt change in grain size or organic carbon was observed in this core, it is likely that a historical change in organic sedimentation took place during deposition across this time interval.
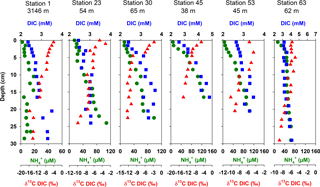
3.4 Pore-water DIC, NH, and δ13CDIC
Pore-water concentrations of DIC and NH increased with depth at all stations (Fig. 4). The increase in DIC was between 0.6 mM (Station 23) and 2.3 mM (Station 45) over the cored sediment depths. Ammonium concentrations increased between 16.8 (Station 1) and 549 µM (Station 50). The steepness of the depth gradients was consistent with the rates of oxygen uptake and bacterial sulfate reduction for the different stations. The pore-water pattern at Station 63 was an exception because this station had the highest oxygen uptake and the highest sulfate reduction rates of all stations but showed only a modest increase in DIC and NH concentrations of 1.5 mM and 57 µM, respectively, over the cored sediment depth. The low DIC concentrations are consistent with the very low rates of sulfate reduction below 10 cm depth. Since these deeper layers have not produced large amounts of DIC and NH, only the surface 10 cm contribute significantly to total carbon mineralization and ammonium production.
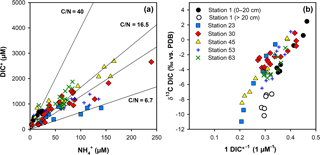
Figure 5(a) Cross plot of dissolved NH and pore-water DIC* after correction for bottom-water DIC concentrations. The average pore-water DIC ∕ NH ratios for the individual stations are shown in Table 2. (b) Keeling plot of the fraction of remineralized DIC calculated from a two-end-member mixing model versus δ13CDIC.
For the anoxic parts of the sediment, DIC ∕ NH ratios varied between 8.4 for Station 24 and 40 for Station 1 in the Laptev Sea and between 7.2 and 18.8 in the East Siberian Sea, with an overall mean DIC ∕ NH4 ratio of 9.8 for all stations, excluding the continental slope stations (Fig. 5a). The δ13C values of DIC consistently decreased with sediment depth, indicating the addition of 13C-depleted remineralized carbon to DIC. The greatest down-core depletion in 13C was observed at Stations 45, 48, and 50, where δ13C of DIC decreased from −2.0 ‰ near the sediment surface to −13.9, −16.4, and −18.6 ‰ at the bottom of the cores (Fig. 4).
3.5 Benthic exchange and modeled O2, iron, and manganese reduction rates
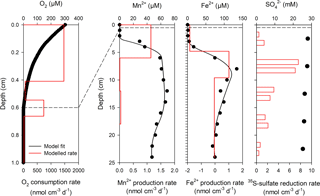
Benthic exchange fluxes from whole-core incubations and modeled DIC fluxes are shown in Table 2. There was a good agreement between 2-D O2 optode flux measurements and modeled fluxes, whereas modeled DIC fluxes were generally lower than whole-core incubation fluxes. We attribute this discrepancy to an underestimation of the DIC gradient at the sediment–water interface. Examples of the reaction transport modeling results for dissolved O2, iron, and manganese are shown in Fig. 6 (Station 23). Optimal fits of the concentration profiles required a bioirrigation coefficient of 1 × 10−4 cm2 s−1 in the topmost 2 cm of sediment at Stations 23 and 53. For the other stations, optimal fits required no sediment mixing by bioturbation or advective pore-water transport by bioirrigation. This is consistent with the low numbers of bioturbating macrofauna in the outer shelf sediment. O2 consumption rates exceeded sulfate and net reduction rates of iron and manganese by a factor of 100 (Fig. 6). On the shelf, most of the carbon oxidation therefore takes place in the topmost 5 mm. The reaction rate depth profiles for iron and manganese indicate that manganese reduction dominates in the topmost 2 cm of sediment followed by coexisting iron and sulfate reduction below (Fig. 6). Bacterial sulfate reduction was detected at a depth at which the sediment was still brown, indicating abundant iron and manganese hydroxides. The modeled negative iron production rates at the sediment surface indicate net iron oxidation by oxygen in the mixed upper sediment layer. This pattern was not observed for manganese, which is consistent with incomplete manganese oxidation at the sediment surface and loss of dissolved manganese to the bottom water.
4.1 Coupled terminal electron-accepting processes
The modeled efflux of manganese to the bottom water on the East Siberian Sea shelf supports results from Macdonald and Gobeil (2012) that Arctic shelf sediments export dissolved manganese to seawater and to the Arctic interior. Coexistence of net iron reduction and sulfate reduction at the same depths make it difficult to quantify how much of the iron reduction is coupled to heterotrophic carbon oxidation as opposed to the reoxidation of sulfide produced from bacterial sulfate reduction. The presence of dissolved iron throughout the measured pore-water profiles implies that iron reduction exceeded concomitant sulfate reduction, iron sulfide precipitation, and reoxidation reactions, which supports the assessment of net heterotrophic iron reduction. Assuming minor direct redox coupling between the TEAPs, manganese and iron reduction contributed maximally between 2.3 and 23.7 % to the total anaerobic carbon mineralization and between 0.3 and 2.3 % to the total carbon mineralization (Table 3). Even if our calculations only approximate the true contribution of metals to carbon mineralization, the results indicate that bacterial sulfate reduction is by far the major anaerobic carbon mineralization pathway in these sediments. The prevalence of bacterial sulfate reduction in anaerobic carbon mineralization agrees with results of Vandieken et al. (2006) and Nickel et al. (2008) from the northern Barents Sea where ice-free stations support higher rates of sulfate reduction than the more permanently ice-covered stations, reflecting lower carbon export production.
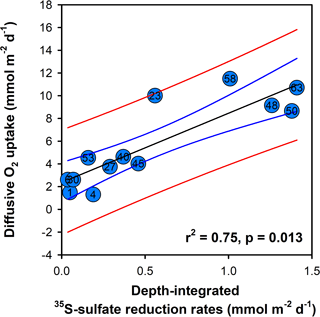
Figure 7Cross plot of diffusive oxygen uptake and integrated sulfate reduction rates. The black line is the result of the regression analysis that yielded a y intercept of 2.1 mmol m−2 d−1 and a slope of 6.1 ± 0.1. Blue and red lines show the 95 and 99 % confidence intervals.
The significant correlation (r2 0.75, P < 0.05) between the dissolved O2 uptake and anaerobic carbon degradation by sulfate reduction with a slope of 6.1 ± 1.1 (Fig. 7) reflects the coupling of oxygen uptake to the oxidation of reduced inorganic metabolites (FeS and H2S) produced during sulfate reduction (e.g., Glud, 2008; Jørgensen and Kasten, 2006; Thamdrup, 2000; Berg et al., 2003). The percentage of the inverse of this slope, 16.4 %, is slightly lower than the 23 % reported for oxygenated coastal and continental shelf sediment (Canfield et al., 2005) but is consistent with the notion that a substantial amount of the buried organic matter in Siberian shelf sediment is oxidized anaerobically. The lower proportion of anaerobic respiration compared to other shelf environments likely reflects the greater proportion of highly reactive marine-derived organic material in the topmost millimeters of sediment.
4.2 Marine versus terrestrial organic matter contribution
Terrestrial organic carbon sources to the Laptev and East Siberian shelf and slope are riverine discharge and coastal erosion of the ice core complex (Stein and Macdonald, 2004; Vonk et al., 2012; Rachold et al., 2004; Fahl and Nöthig, 2007; Semiletov, 1999). Marine organic carbon is derived from open-water production during the ice-free months, export of ice algae, and new production in polynyas (Sakshaug et al., 2004; Nitishinsky et al. 2007). Generally, marine productivity in the Laptev Sea is low and controlled by the nutrients derived from Atlantic water, but spring outflow from the Lena River provides an additional temporary land-derived nutrient source (Pivovarov et al., 1999; Sakshaug et al., 2004; Nitishinsky et al., 2007; Bourgeois et al., 2017) during late spring ice melt (Raymond et al., 2007). Terrestrially derived nutrients can also directly affect marine productivity by new production, or indirectly due to plankton production from remineralized, terrestrially derived dissolved organic carbon and particulate organic carbon (Alling et al., 2012; Tesi et al., 2017). In the eastern East Siberian and Chukchi seas, the inflow of nutrient-rich Pacific water supports higher marine primary productivity (e.g., Grebmeier et al., 2006). Ice-rafted transport and bottom boundary layer transport are the two most important modes of particle transport (Wegner et al., 2005; Bauch et al., 2009). Since all sediments sampled in this study were finely grained silty clays and clayey silts, coarse-grained woody, ice-rafted material plays only a minor role for deposition of organic matter on the outer shelf and slope sediment. The transport direction of inner-shelf sediments may follow the predominant atmospheric regime, which is thought to be linked to the Arctic Oscillation (Weingartner et al., 1999; Guay et al., 2001; Dimitrenko et al., 2008). During positive Arctic Oscillation southwesterly winds lead to generally eastward transport and repeated inshore transport in the benthic boundary layer, whereas a negative Arctic Oscillation favors southerly winds and a predominantly northward transport (Guay et al., 2001; Dmitrenko et al., 2008). Offshore transport of dissolved and particulate organic matter from the Lena delta to the north can occur with the Transpolar Drift, but terrestrial organic material is also transported eastward and obliquely offshore with the Siberian coastal current, receiving additional organic material from the Indigirka and Kolyma rivers (Dudarev et al., 2006; Guo et al., 2007). East of 140∘ E, the influence of Pacific-derived nutrient-rich water supporting marine production is stronger the further east and offshore the sampling stations are located (Semiletov et al., 2005; Fig. 1).
Carbon degradation rates in the sediment across the whole Siberian shelf and slope reflect this temporally and spatially diverse distribution of nutrient availability, ice cover, sediment deposition, and current flow regime (Rachold et al., 2004; Dudarev et al., 2006; Semiletov et al., 2005; Sakshaug et al., 2004; Dmitrenko et al., 2005). The proportion of degradable marine-derived organic material at eastern Stations 50 to 63 on the East Siberian shelf is higher than at the western stations in the Laptev Sea, in line with higher nutrient availability due to the Pacific influence. Ice-free conditions and the opening of water due to northward migration of ice shortly before the sampling likely supported new algal primary production at the shelf stations closest to land, leading to enhanced export and deposition on the seafloor. During the time of sampling, only Stations 6 to 27 were ice free, while Stations 23 and 24 had the longest ice-free condition before sampling. By contrast, Stations 30 to 63 were covered by ice during sampling. New export of reactive organic material explains why O2 uptake rates were the highest at Stations 23 and 24 along the shelf-to-slope transect from Station 1 to Station 24 (Boetius and Damm, 1998). The same pattern as for the O2 uptake rates is also observed for the sulfate reduction rates, indicating that reactive organic matter is also buried below the oxygen penetration depth and mixed sediment layer into the sulfate-reducing zone. This indicates that a greater portion of reactive organic material is buried closer to the Lena delta.
Table 4Calculated carbon isotope composition of remineralized DIC and mass fractions of the marine and terrestrial end-members and corresponding terrestrial carbon degradation rates based on 35S-SRR and DIC flux.
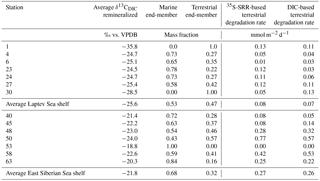
Derivation of the carbon isotope composition of the remineralized organic matter using Keeling plots (Fig. 5b) yielded δ13C values of remineralized DIC between −18.8 ‰ ± 1.1 ‰ (Station 53) and −35.8 ‰ ± 3.0 ‰ in the lower sediment section of Station 1 (Table 4). In the top 20 cm of sediment at Station 1, the regression yielded an isotope composition of −22.7 ‰ for remineralized DIC. This latter isotopic composition reflects the bulk organic matter composition of sedimentary organic carbon at Station 1, which is −22.3 ‰ (Table 2; Salvado et al., 2015). The more depleted values below 20 cm depth suggest that an isotopically distinct fraction of organic matter fuels carbon degradation in the buried sediments. Salvado et al. (2016) report terrestrially derived lignin from sediment at this station pointing to the presence of terrestrial organic matter. Terrestrially derived n-alkanes can have such low δ13C values. Slow degradation of terrestrially derived lipids in these sediments suggests a contribution of terrestrial organic matter to carbon mineralization in slope sediment, in line with the very high DIC ∕ NH ratio of the pore waters (Fig. 5a). The contribution of degradable terrestrial organic matter to DIC in slope sediments is also supported by the observation of terrestrially derived biomarkers in pore-water DOC of central Arctic Ocean sediment analyzed with Fourier transform inductively coupled ion cyclotron resonance mass spectrometry (FT-ICRMS; Rossel et al., 2016) and deep-water sediment trap data in the central Arctic Ocean (Fahl and Nöthig, 2007) but requires further investigation. Our isotope mass balance calculations indicate that in the Laptev Sea on average 47 % of the remineralized DIC is derived from terrestrial organic carbon. This proportion decreases to an average value of 32 % in the East Siberian Sea, in line with a greater marine production in this area due to the inflow of Pacific water (Semiletov et al., 2005; Dudarev et al., 2006; Naidu et al., 2000).
Published organic carbon budgets for the Arctic shelves infer an average burial efficiency of about 1 % of exported marine organic carbon (Stein and Macdonald, 2004), while terrestrial organic carbon, accounting for about 10 % of the organic carbon delivered to the Arctic Ocean bottom, has been suggested to be preserved with about 90 % efficiency (Macdonald et al., 2015). In contrast, Semiletov et al. (2015) compiled a large data set indicating substantial aragonite undersaturation of Arctic shelf bottom waters from the Laptev Sea, the East Siberian Sea, and the Russian part of the Chukchi Sea and inferred widespread remineralization of terrestrial organic matter in the bottom waters and sediments. The observation of strongest aragonite undersaturation in the bottom waters supports a sediment-derived CO2 source or a stagnant bottom boundary layer (Semiletov et al., 2013). It is therefore possible that oxic carbon mineralization in the topmost millimeter of sediment is a major CO2 source for the overlying water.
Since 35S-sulfate reduction rates comprise most of the anaerobic carbon mineralization of sediment buried below the oxygen penetration depth, our assessment includes, in contrast to earlier studies, an assessment of terrestrial organic matter mineralization rates beyond the short time period of oxygen exposure in the topmost millimeter of sediment. Using shelf sedimentation rates of 0.8 mm yr−1 for the outer Laptev Sea (Strobl et al., 1988) and 1.4 mm yr−1 for the outer East Siberian Sea (Bröder et al., 2016b), the recovered shelf sediments record a time interval of 250 to 700 years since burial with oxygen exposure times between 2 and 45 years. The mineralization rates of the terrestrial and marine carbon fractions were derived from the product of the mass fractions and the depth-integrated anaerobic carbon mineralization rates (Table 4). This approach is only applicable for depth-integrated anaerobic carbon mineralization rates but not for oxic carbon mineralization because the depth of oxygen penetration and the DIC measurements cover different depth intervals. The proportions calculated based on the δ13C composition of DIC deeper in the sediment do not necessarily apply to the topmost millimeter of sediment. It is therefore not possible to relate the relative fractions of mineralized terrestrial and marine organic matter to discrete depth intervals in the oxic zone.
The gradient in carbon mineralization rates measured between Stations 1 and 24 reflects the influence of gradual offshore transport of terrestrial organic material (Bröder et al., 2016a; Fig. 8a, b). Oxygen uptake rates reported by Boetius and Damm (1998) measured in the early fall of 1993 ranged between 0.16 and 1.56 mmol m−2 d−1. The rates in our 2014 study (0.81 to 11.49 mmol m−2 d−1) cover the same water depth range and are significantly higher than those measured in 1993 by Boetius and Damm (1998) using a similar methodology (Student's t test < 0.01). To some extent, the different rates may reflect seasonal differences because Boetius and Damm (1998) acquired their data later in the fall. However, the increase may also point to higher organic carbon mass accumulation rate compared to 20 years ago, consistent with a decrease in the annual ice cover over the past 20 years in the Arctic (Arrigo and van Dijken, 2011). The data cannot answer whether the higher rates reflect an increase in marine and/or terrestrial accumulation.
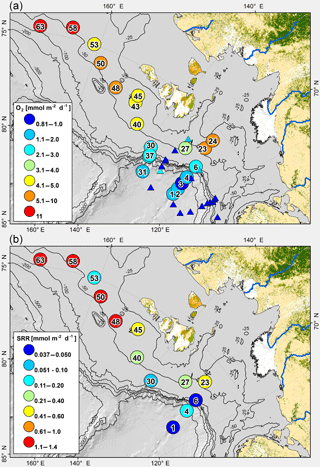
Figure 8(a) Map of field area and sampling stations showing oxygen uptake rates. For comparison, oxygen uptake rates reported in Boetius and Damm (1998) are shown as triangles using the same color code. (b) Map of field area and sampling stations with depth-integrated sulfate reduction rates.
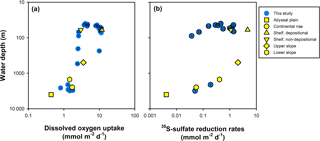
Figure 9(a) Water depth variation in sediment oxygen uptake. (b) Water depth variation in integrated 35S-sulfate reduction rates (0–30 cm sediment depth). For reference average rates of abyssal plain, continental rise, slope, and shelf sediments, depositional and non-depositional shelf sediments are shown for reference.
Figure 9a compares the oxygen uptake rate of the stations of this study with average oxygen uptake rates from the literature for different shelf, slope, and abyssal plain environments worldwide (Canfield et al., 2005). There is no significant difference in the oxygen consumption rates between the Siberian shelf and slope and other continental margin environments (Student's t test > 0.5). 35S-sulfate reduction rates in these East Siberian slope sediments are also comparable to those in other slope environments (Fig. 9b), except for the shelf, where reduction rates are up to a factor of 15 lower. The apparent similarity in sulfate reduction rates for the outer shelf and continental slope sediments of the Siberian Arctic suggests similar reactivity of the organic matter. This is surprising since accumulation rates on the continental slope are probably lower than on the shelf. Accumulation of the organic material on the slope may therefore be related to rapid downslope transport of organic material or rapid offshore transport, e.g., due to transport with ice or as bottom nepheloid layers cascading from the shelf edge (Ivanov and Golovin, 2007).
4.3 Assessment of carbon burial efficiency
Reported 210Pb-based sediment accumulation rates in outer Siberian shelf sediment range between 0.05 ± 0.02 g cm−2 yr−1 in the Laptev Sea (Strobl et al., 1988) and 0.24 ± 0.04 g cm−2 yr−1 in the East Siberian Sea (Bröder et al., 2016b). Given surface sediment organic carbon concentrations for this area of between 1 and 1.5 %, the resulting organic carbon mass accumulation rates vary between 1.1 and 1.7 mmol m−2 d−1 for the Laptev Sea (area near Station 23) and between 5.5 and 8.2 mmol m−2 d−1 in the East Siberian Sea (data for Station 63). A comparison with the total oxygen uptake indicates that the 210Pb-based Corg accumulation rates on the shelf are equal to or significantly lower than the oxygen uptake rates, with a discrepancy of up to a factor of 10. Since the 210Pb data cover the same depth range as our direct degradation rate measurements, Corg mass accumulation rates and anaerobic degradation rate measurements cover the same time window of sediment burial. Temporal variation in sediment accumulation therefore cannot explain the discrepancy. The best explanation is that the 210Pb mass accumulation rates underestimate the true Corg mass accumulation rate. Labile organic carbon is oxidized at the sediment surface and is not adequately accounted for in the Corg measurements, likely due to coarse sediment sampling resolution. A better account of the true mass accumulation of organic carbon is therefore the sum of the oxygen uptake converted to CO2 equivalents plus the 210Pb-based Corg mass accumulation.
We estimated the total burial efficiency of organic carbon from the sum of the depth-integrated aerobic and anaerobic degradation relative to the sum of the CO2 equivalent from O2 uptake and the 210Pb mass accumulation rate of organic carbon. The resulting burial efficiency of the total organic carbon is on average 28 ± 10 % in the Laptev Sea and 52 ± 11 % for the East Siberian Sea. Based on the measured oxygen uptake rates, this freshly deposited organic material has substantially higher degradation rates within the top millimeter of sediment, as reflected by the steep O2 gradients. It is likely that this labile fraction consists of more marine than terrestrial organic matter, but the degradation kinetics of these two pools in the oxic zone cannot be assessed reliably with these measurements.
4.4 Regional estimates
We present areal estimates of sediment carbon mineralization by extrapolating the measured carbon mineralization rates over the outer Laptev Sea and East Siberian Sea shelf. Such extrapolations of benthic carbon mineralization rates are notoriously difficult given sediment heterogeneity and insufficient temporal data coverage of benthic carbon mineralization rates. For this investigation, no near-shore or slope stations were included in the assessment. The near-shore Siberian shelf environments are under a much stronger influence of coastal erosion and riverine discharge than the outer shelf stations and have considerably longer open-water conditions than the outer shelf stations investigated here. In addition, the sedimentation pattern in the near-shore environments is significantly more diverse, which would affect sedimentation rates, grain size distribution, and carbon contents. For this reason, we did not extend our extrapolations to the inner-shelf environments. Some of these inner-shelf settings likely have much higher benthic carbon mineralization rates and additional studies are required to constrain these better. Our coverage of the slope stations is insufficient for meaningful spatial extrapolations.
Table 5Regional estimates of sediment carbon mineralization in the outer Laptev and East Siberian sea shelf.
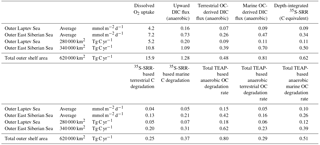
We estimate the extent of the outer shelf area with depositional conditions comparable to those investigated here to cover approximately 280 000 km2 of the Laptev Sea. For the East Siberian Sea, we estimate the respective area of the outer shelf to be 340 000 km2. Due to the stronger terrestrial influence in the Laptev Sea, we calculated rates separately for the two shelf seas. The areal coverage with sediment stations was too sparse for statistically significant interpolations between stations to give reliable spatial accounts of the gradients in rates between the stations. Instead, arithmetic averages of sediment mineralization rates and fluxes were calculated for these regions. Accepting the uncertainties in our assessment, we estimate that the calculated areal rates could deviate by up to 50 %. Table 5 lists the calculated rates based on the average flux calculated per square meter per day for oxygen uptake, DIC flux, bacterial sulfate reduction, and total anaerobic carbon mineralization. Our O2 uptake calculations suggest that 5.2 and 10.4 Tg C yr−1 is released from outer shelf sediment in the Laptev and East Siberian seas, respectively, totaling 15.9 Tg C yr−1 for the whole investigated area (Table 5). The rates calculated with our data set agree well with the O2 uptake rates recently published by Bourgeois et al. (2017) for the Laptev Sea. Anaerobic carbon mineralization based on DIC, 35S-SRR, and combined manganese, iron, and sulfate reduction range between 0.62 and 1.28 Tg C yr−1. Between 0.25 and 0.48 Tg C yr−1 is attributed to the anaerobic oxidation of terrestrially derived organic material, which represents 0.5 to 8 % of the annual terrestrial organic matter load to the Laptev and East Siberian seas, ranging from 6 Tg yr−1 (Stein and Macdonald, 2004) to 22 ± 8 Tg yr−1 (Vonk et al., 2012). The anaerobic degradation is thus 5 to 10 times lower than the estimated annual water column degradation of particulate terrestrial organic matter in the eastern Siberian Arctic shelf system of 2.5 ± 1.6 Tg C yr−1 (Sanchez et al., 2011) and represents between 0.5 and 2 % of the annual organic carbon export from land.
There are large uncertainties associated with these estimates, given that our calculations do not account for carbon mineralization of resuspended terrestrial organic material and likely higher rates of mineralization in inner-shelf sediments. Nevertheless, these data indicate that the contribution of the benthic DIC flux to the total CO2 production in the outer East Siberian Sea and Laptev Sea is small. This conclusion, however, does not necessarily extend to the inner parts of the Laptev Sea and the western parts of the East Siberian Sea, where CO2 supersaturation has been reported by Pipko et al. (2011). Anderson et al. (2009) estimated a DIC excess of 10 Tg C by evaluating data from the Laptev and East Siberian seas collected in the summer of 2008 and suggested that this excess was caused mainly by terrestrial organic matter decomposition. Their estimate can be compared to our sediment oxygen uptake for the outer Laptev and East Siberian sea shelf of almost 16 Tg O2 yr−1, which would demand that 62.5 % of the oxygen uptake was due to terrestrial organic matter mineralization. However, the reported annual production of marine organic matter for the total Laptev Sea and East Siberian Sea is about 46 Tg C yr−1 (Stein and Macdonald, 2004). Even if only half of this amount is produced in the outer shelf region and another half of that amount is deposited on the seafloor, there would still be more than 10 Tg C yr−1 of reactive marine organic matter available at the sediment surface. Our data would therefore suggest that, at least in the more productive East Siberian Sea, the pronounced aragonite undersaturation reported for bottom waters is probably due to aerobic mineralization of largely marine organic matter with a lesser contribution from terrestrial organic matter.
In Laptev and East Siberian sea shelf and slope sediment between 47 and 32 % of the DIC in pore waters is derived from terrestrial organic matter mineralization, which occurs largely by bacterial sulfate reduction. This conclusion confirms previous observations that terrestrial organic carbon buried in Siberian shelf and slope sediment is not conservative (Semiletov et al., 2013; Karlsson et al., 2015; Bröder et al., 2016b). While mineralization of terrestrial organic material has been reported for the shelf waters and resuspended surface sediment, our data extend this observation to anaerobic degradation in buried sediment. When compared to estimates of the terrestrial organic carbon load to the shelf, terrestrial carbon mineralization in buried sediment under anaerobic conditions is minor. Mass balance considerations suggest that the greater part of the oxygen consumption in the top centimeter of sediment is due to marine organic matter mineralization and that the degrading marine proportion in the oxic zone is higher than in the anaerobic zone. Further studies are required to better quantify the contribution of terrestrial carbon mineralization under aerobic conditions. Future changes in terrestrial organic carbon load and marine production on the Siberian shelf under longer ice-free conditions (Arrigo and van Dijken, 2011) will likely change the present-day proportions of degrading marine and terrestrial organic matter further. This requires continued long-term studies of carbon mineralization at the seafloor to assess the effect of sediment carbon mineralization for the CO2 inventory of this shallow shelf sea.
All data from this publication are deposited in the database of the Bolin Centre for Climate Research: https://bolin.su.se/data and searchable under Brüchert, Biogeosciences.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-15-471-2018-supplement.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Climate–carbon–cryosphere interactions in the East Siberian Arctic Ocean: past, present and future (TC/BG/CP/OS inter-journal SI)”. It is not associated with a conference.
Funding for this investigation came from the K&A Wallenberg foundation,
the Swedish Polar Research Secretariat, and the Bolin Centre for Climate Research at
Stockholm University. Igor Semiletov acknowledges support from the Russian
Government (no. 14.Z50.31.0012/03.19.2014). We would like to thank the
members of the SWERUS-C3 consortium, the ship crew on the icebreaker Oden, and
Heike Siegmund, Lina Hansson, Barkas Charalampos, and Dimitra Panagiotopoulou for help with the laboratory work. We dedicate this
publication to our friend and colleague Vladimir Samarkin, who unfortunately
passed away before publication of this work. This paper benefitted from
discussions with Patrick Crill, Rienk Smittenberg, Örjan Gustafsson,
Christoph Humborg, Julia Steinbach, Clint Miller, Marc Geibel, Emma Karlsson, Brett Thornton, Jorien Vonk, Leif Anderson, and Magnus Mörth.
Edited by: Francien Peterse
Reviewed by: two anonymous referees
Alling, V., Porcelli, D., Mörth, C. M., Anderson, L. G., Sanchez-Garcia, L., Gustafsson, Ö., Andersson, P. S., and Humborg, C.: Degradation of terrestrial organic carbon, primary production and out-gassing of CO2 in the Laptev and East Siberian Seas as inferred from δ13C values of DIC, Geochim. Cosmochim. Ac., 95, 143–159, 2012.
Anderson, L. G., Jutterström, S., Hjalmarsson, S., Wahlstrom ,I., and Semiletov, I. P.: Out-gassing of CO2 from Siberian Shelf seas by terrestrial organic matter decomposition, Geophys. Res. Lett., 36, L20601, https://doi.org/10.1029/2009gl040046, 2009.
Arrigo, K. R. and van Dijken, G. L.: Secular trends in Arctic Ocean net primary production, J. Geophys. Res.-Oceans, 116, C09011, https://doi.org/10.1029/2011JC007151, 2011.
Bauch, D., Dmitrenko, I., Kirillov, S., Wegner, C., Hölemann, J., Pivovarov, S., Timokhov, L., and Kassens, H.: Eurasian Arctic shelf hydrography: Exchange and residence time of southern Laptev Sea waters, Cont. Shelf Res., 29, 1815–1820, 2009.
Berg, P., Petersen-Risgaard, N., and Rysgaard, S.: Interpretation and measured concentration profiles in sediment pore water, Limnol. Oceanogr., 43, 1500–1510, 1998.
Berg, P., Rysgaard, S., and Thamdrup, B.: Dynamic modeling of early diagenesis and nutrient cycling. A case study in an Arctic marine sediment, Am. J. Sci., 303, 906–955, 2003.
Boetius, A. and Damm, E.: Benthic oxygen uptake, hydrolytic potentials and microbial biomass at the Arctic continental slope, Deep-Sea Res. Pt. I, 45, 239–275, 1998.
Boudreau, B. P.: Diagenetic models and their implementation, Springer Verlag, 414 pp., 1996.
Bourgeois, S., Archambault, P., and Witte, U.: Organic matter remineralization in marine sediments: A Pan-Arctic synthesis, Global Biogeochem. Cy., 31, 190–213, 2017.
Bröder, L., Tesi, T., Salvadó, J. A., Semiletov, I. P., Dudarev, O. V., and Gustafsson, Ö.: Fate of terrigenous organic matter across the Laptev Sea from the mouth of the Lena River to the deep sea of the Arctic interior, Biogeosciences, 13, 5003–5019, https://doi.org/10.5194/bg-13-5003-2016, 2016a.
Bröder, L., Tesi, T., Andersson, A., Eglinton, T. I., Semiletov, I. P., Dudarev, O. V., Roos, P., and Gustafsson, Ö.: Historical records of organic matter supply and degradation status in the East Siberian Sea, Org. Geochem., 91, 16–30, 2016b.
Canfield, D. E., Kristensen, E., and Thamdrup, B.: Aquatic geomicrobiology, Adv. Mar. Biol., 48, 640 pp., 2005.
Dmitrenko, I. A., Tyshko, K. N., Kirillov, S. A., Eicken, H., Hölemann, J. A., and Kassens, H.: Impact of flaw polynyas on the hydrography of the Laptev Sea, Global Planet. Change, 48, 9–27, 2005.
Dmitrenko, I. A., Kirillov, S. A., and Tremblay, L. B.: The long-term and interannual variability of summer fresh water storage over the eastern Siberian shelf: Implication for climatic change, J. Geophys. Res.-Oceans, 113, C03007, https://doi.org/10.1029/2007JC004304, 2008.
Dudarev, O. V., Semiletov, I. P., and Charkin, A. N.: Particulate material composition in the Lena River-Laptev Sea system: Scales of heterogeneities, in: Doklady Earth Sciences, 6, 1000–1005, 2006.
Fahl, K. and Nöthig, E.-M.: Lithogenic and biogenic particle fluxes on the Lomonosov Ridge (central Arctic Ocean) and their relevance for sediment accumulation: Vertical vs. lateral transport, Deep-Sea Res. Pt. I, 54, 1256–1272, 2007.
Glud, R. N.: Oxygen dynamics of marine sediments, Mar. Biol. Res., 4, 243–289, 2008.
Grebmeier, J. M., Cooper, L. W., Feder, H. M., and Sirenko, B. I.: Ecosystem dynamics of the Pacific-influenced Northern Bering and Chukchi Seas in the Amerasian Arctic, Prog. Oceanogr., 71, 331–361, 2006.
Guay, C. K., Falkner, K. K., Muench, R. D., Mensch, M., Frank, M., and Bayer, R.: Wind-driven transport pathways for Eurasian Arctic river discharge, J. Geophys. Res.-Oceans, 106, 11469–11480, 2001.
Guo, L., Ping, C. L., and Macdonald, R. W.: Mobilization pathways of organic carbon from permafrost to arctic rivers in a changing climate, Geophys. Res. Lett., 34, L13603, 2007.
Hall, P. O. J. and Aller, R. C.: Rapid, small-volume flow injection analysis for ΣCO2 and NH in marine and freshwaters, Limnol. Oceanogr., 37, 1113–1119, 1992.
Hugelius, G., Strauss, J., Zubrzycki, S., Harden, J. W., Schuur, E. A. G., Ping, C.-L., Schirrmeister, L., Grosse, G., Michaelson, G. J., Koven, C. D., O'Donnell, J. A., Elberling, B., Mishra, U., Camill, P., Yu, Z., Palmtag, J., and Kuhry, P.: Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps, Biogeosciences, 11, 6573–6593, https://doi.org/10.5194/bg-11-6573-2014, 2014.
Ivanov, V. V. and Golovin, P. N.: Observations and modeling of dense water cascading from the northwestern Laptev Sea shelf, J. Geophys. Res.-Oceans, 112, C09003, https://doi.org/10.1029/2006jc003882, 2007.
Jørgensen, B. B.: A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments: I. Measurement with radiotracer techniques, Geomicrobiol. J., 1, 11–27, 1978.
Jørgensen, B. B. and Kasten, S.: Sulfur and methane oxidation, in: Marine Geochemistry, 2nd Edn., edited by: Schulz, H. D. and Zabel, M., Springer Verlag, Berlin Heidelberg, 271–309, 2006.
Kallmeyer, J., Ferdelman, T. G., Weber, A., Fossing, H., and Jørgensen, B. B.: Evaluation of a cold chromium distillation procedure for recovering very small amounts of radiolabeled sulfide related to sulfate reduction measurements, Limnol. Oceanogr.-Methods, 2, 171–180, 2004.
Karlsson, E. S., Brüchert, V., Tesi, T., Charkin, A., Dudarev, O., Semiletov, I., and Gustafsson, Ö.: Contrasting regimes for organic matter degradation in the East Siberian Sea and the Laptev Sea assessed through microbial incubations and molecular markers, Mar. Chem., 170, 11–22, 2015.
Koven, C. D., Lawrence, D. M., and Riley, W. J.: Permafrost carbon-climate feedback is sensitive to deep soil carbon decomposability but not deep soil nitrogen dynamics, P. Natl. Acad. Sci. USA, 112, 3752–3757, 2015.
Li, Y.-H. and Gregory, S.: Diffusion of ions in sea water and in deep-sea sediments, Geochim. Cosmochim. Ac., 88, 703–714, 1974.
Macdonald, R. W. and Gobeil, C.: Manganese Sources and Sinks in the Arctic Ocean with Reference to Periodic Enrichments in Basin Sediments, Aquat. Geochem., 18, 565–591, 2012.
Macdonald, R. W., Kuzyk, Z. Z. A., and Johannessen, S. C.: The vulnerability of Arctic shelf sediments to climate change, Environ. Rev., 23, 1–19, 2015.
Mackin, J. E. and Aller, R.C.: Ammonium adsorption in marine sediments, Limnol. Oceanogr., 29, 250–257, 1984.
McGuire, A. D., Anderson, L. G., Christensen, T. R., Dallimore, S., Guo, L., Hayes, D. J., Heimann, M., Lorenson, T. D., Macdonald, R. W., and Roulet, N.: Sensitivity of the carbon cycle in the Arctic to climate change, Ecol. Monogr., 79, 523–555, 2009.
Naidu, A. S., Cooper, L. W., Finney, B. P., Macdonald, R. W., Alexander, C., and Semiletov, I. P.: Organic carbon isotope ratios (δ13C) of Arctic Amerasian Continental shelf sediments, Int. J. Earth Sci., 89, 522–532, 2000.
Nickel, M., Vandieken, V., Brüchert, V., and Jørgensen, B. B.: Microbial Mn(IV) and Fe(III) reduction in northern Barents Sea sediments under different conditions of ice cover and organic carbon deposition, Deep-Sea Res. Pt. II, 55, 2390–2398, 2008.
Nitishinsky, M., Anderson, L. G., and Hölemann, J. A.: Inorganic carbon and nutrient fluxes on the Arctic Shelf, Cont. Shelf Res., 27, 1584–1599, 2007.
Pipko, I. I., Semiletov, I. P., Pugach, S. P., Wåhlström, I., and Anderson, L. G.: Interannual variability of air-sea CO2 fluxes and carbon system in the East Siberian Sea, Biogeosciences, 8, 1987–2007, https://doi.org/10.5194/bg-8-1987-2011, 2011.
Pivovarov, S., Hölemann, J., Kassens, H., Antonow, M., and Dmitrenko, I.: Dissolved oxygen, silicon, phosphorous and suspended matter concentrations during the spring breakup of the Lena River, in: Land–Ocean Systems in the Siberian Arctic: Dynamics and History, edited by: Kassens, H., Bauch, H. A., Dmitrenko, I., Eicken, H., Hubberten, H.-W., Melles, M., Thiede, J., and Timokhov, L., Springer, Berlin, 251–264, 1999.
Rachold, V., Eicken, H., Gordeev, V., Grigoriev, M. N., Hubberten, H.-W., Lisitzin, A. P., Shevchenko, V., and Schirrmeister, L.: Modern terrigenous organic carbon input to the Arctic Ocean, in: The organic carbon cycle in the Arctic Ocean, Springer, 33–55, 2004.
Rasmussen, H. and Jørgensen, B. B.: Microelectrode studies of seasonal oxygen uptake in a coastal sediment: Role of molecular diffusion, Mar. Ecol.-Prog. Ser., 81, 289–303, 1992.
Raymond, P. A., McClelland, J., Holmes, R., Zhulidov, A., Mull, K., Peterson, B., Striegl, R., Aiken, G., and Gurtovaya, T.: Flux and age of dissolved organic carbon exported to the Arctic Ocean: A carbon isotopic study of the five largest arctic rivers, Global Biogeochem. Cy., 21, GB4017, https://doi.org/10.1029/2007GB002983, 2007.
Rossel, P. E., Bienhold, C., Boetius, A., and Dittmar, T.: Dissolved organic matter in pore water of Arctic Ocean sediments: Environmental influence on molecular composition, Org. Geochem., 97, 41–52, 2016.
Sakshaug, E.: Primary and secondary production in the Arctic Seas, in: The organic carbon cycle in the Arctic Ocean, Springer, 57–81, 2004.
Salvadó, J. A., Tesi, T., Andersson, A., Ingri, J., Dudarev, O. V., Semiletov, I. P., and Gustafsson, Ö.: Organic carbon remobilized from thawing permafrost is resequestered by reactive iron on the Eurasian Arctic Shelf, Geophys. Res. Lett., 42, 8122–8130, 2015.
Sánchez-García, L., Alling, V., Pugach, S., Vonk, J., van Dongen, B., Humborg, C., Dudarev, O., Semiletov, I., and Gustafsson, Ö.: Inventories and behavior of particulate organic carbon in the Laptev and East Siberian seas, Global Biogeochem. Cy., 25, Gb2007, https://doi.org/10.1029/2010gb003862, 2011.
Savvichev, A., Rusanov, I., Pimenov, N., Zakharova, E., Veslopolova, E., Lein, A. Y., Crane, K., and Ivanov, M.: Microbial processes of the carbon and sulfur cycles in the Chukchi Sea, Microbiology, 76, 603–613, 2007.
Schuur, E. A. G., McGuire, A. D., Schadel, C., Grosse, G., Harden, J. W., Hayes, D. J., Hugelius, G., Koven, C. D., Kuhry, P., Lawrence, D. M., Natali, S. M., Olefeldt, D., Romanovsky, V. E., Schaefer, K., Turetsky, M. R., Treat, C. C., and Vonk, J. E.: Climate change and the permafrost carbon feedback, Nature, 520, 171–179, 2015.
Seeberg-Elverfeldt, J., Schlüter, M., Feseker, T., and Kölling, M.: Rhizon sampling of porewaters near the sediment-water interface of aquatic systems, Limnol. Oceanogr.-Methods, 3, 361–371, 2005.
Semiletov, I., Dudarev, O., Luchin, V., Charkin, A., Shin, K.-H., and Tanaka, N.: The East Siberian Sea as a transition zone between Pacific-derived waters and Arctic shelf waters, Geophys. Res. Lett., 32, L10614, https://doi.org/10.1029/2005GL022490, 2005.
Semiletov, I., Pipko, I., Gustafsson, Ö., Anderson, L. G., Sergienko, V., Pugach, S., Dudarev, O., Charkin, A., Gukov, A., Broder, L., Andersson, A., Spivak, E., and Shakhova, N.: Acidification of East Siberian Arctic Shelf waters through addition of freshwater and terrestrial carbon, Nat. Geosci., 9, 361–365, 2016.
Semiletov, I. P.: Destruction of the coastal permafrost ground as an important factor in biogeochemistry of the Arctic Shelf waters, Trans. (Doklady) Russian Acad. Sci., 368, 679–682, 1999 (in English).
Semiletov, I. P., Pipko, I. I., Shakhova, N. E., Dudarev, O. V., Pugach, S. P., Charkin, A. N., McRoy, C. P., Kosmach, D., and Gustafsson, Ö.: Carbon transport by the Lena River from its headwaters to the Arctic Ocean, with emphasis on fluvial input of terrestrial particulate organic carbon vs. carbon transport by coastal erosion, Biogeosciences, 8, 2407–2426, https://doi.org/10.5194/bg-8-2407-2011, 2011.
Semiletov, I. P., Shakhova, N. E., Pipko, I. I., Pugach, S. P., Charkin, A. N., Dudarev, O. V., Kosmach, D. A., and Nishino, S.: Space-time dynamics of carbon and environmental parameters related to carbon dioxide emissions in the Buor-Khaya Bay and adjacent part of the Laptev Sea, Biogeosciences, 10, 5977–5996, https://doi.org/10.5194/bg-10-5977-2013, 2013.
Stein, R. and Macdonald, R. W.: The Organic Carbon Cycle in the Arctic Ocean, Springer-Verlag, Berlin, 382 pp., 2004.
Strobl, C., Schulz, V., Vogler, S., Baumann, S., Kassens, H., Kubik, P. W., Suter, M., and Mangini, A.: Determination of depositional beryllium-10 fluxes in the area of the Laptev Sea and beryllium-10 concentrations in water samples of high northern latitudes, in: Land-Ocean Systems in the Siberian Arctic: Dynamics and History, edited by: Kassens, H., Bauch, H. A., Dmitrenko, I., Eicken, H., Hubberten, H.-W., Melles, M., Thiede, J., and Timokhov, L., Springer, Berlin, 515–532, 1998.
Tesi, T., Semiletov, I., Hugelius, G., Dudarev, O., Kuhry, P., and Gustafsson, Ö.: Composition and fate of terrigenous organic matter along the Arctic land–ocean continuum in East Siberia: Insights from biomarkers and carbon isotopes, Geochim. Cosmochim. Ac., 133, 235–256, 2014.
Tesi, T., Semiletov, I., Dudarev, O., Andersson, A., and Gustafsson, Ö.: Matrix association effects on hydrodynamic sorting and degradation of terrestrial organic matter during cross-shelf transport in the Laptev and East Siberian shelf seas, J. Geophys. Res.-Biogeo., 121, 731–752, 2016.
Tesi, T., Geibel, M. C., Pearce, C., Panova, E., Vonk, J. E., Karlsson, E., Salvado, J. A., Kruså, M., Bröder, L., Humborg, C., Semiletov, I., and Gustafsson, Ö.: Carbon geochemistry of plankton-dominated samples in the Laptev and East Siberian shelves: contrasts in suspended particle composition, Ocean Sci., 13, 735–748, https://doi.org/10.5194/os-13-735-2017, 2017.
Thamdrup, B.: Bacterial manganese and iron reduction in aquatic sediments, Adv. Microb. Ecol., 16, 41–84, 2000.
Vandieken, V., Nickel, M., and Jørgensen, B. B.: Carbon mineralization in Arctic sediments northeast of Svalbard: (Mn(IV) and Fe(III) reduction as principal anaerobic respiratory pathways, Mar. Ecol.-Prog. Ser., 322, 15–27, 2006.
Vonk, J. E., Sanchez-Garcia, L., van Dongen, B. E., Alling, V., Kosmach, D., Charkin, A., Semiletov, I. P., Dudarev, O. V., Shakhova, N., Roos, P., Eglinton, T. I., Andersson, A., and Gustafsson, O.: Activation of old carbon by erosion of coastal and subsea permafrost in Arctic Siberia, Nature, 489, 137–140, 2012.
Wegner, C., Hölemann, J. A., Dmitrenko, I., Kirillov, S., and Kassens, H.: Seasonal variations in Arctic sediment dynamics – evidence from 1-year records in the Laptev Sea (Siberian Arctic), Global Planet. Change, 48, 126–140, 2005.
Wegner, C., Bauch, D., Hölemann, J. A., Janout, M. A., Heim, B., Novikhin, A., Kassens, H., and Timokhov, L.: Interannual variability of surface and bottom sediment transport on the Laptev Sea shelf during summer, Biogeosciences, 10, 1117–1129, https://doi.org/10.5194/bg-10-1117-2013, 2013.
Weingartner, T. J., Danielson, S., Sasaki, Y., Pavlov, V., and Kulakov, M.: The Siberian Coastal Current: A wind- and buoyancy-forced Arctic coastal current, J. Geophys. Res.-Oceans, 104, 29697–29713, 1999.