the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Grazing increases litter decomposition rate but decreases nitrogen release rate in an alpine meadow
Yi Sun
Xiong Z. He
Fujiang Hou
Zhaofeng Wang
Shenghua Chang
Litter decomposition and N release are the key processes that strongly determine the nutrient cycling at the soil–plant interface; however, how these processes are affected by grazing or grazing exclusion in the alpine grassland ecosystems on the Qinghai-Tibetan Plateau (QTP) is poorly understood. So far few studies have simultaneously investigated the influence of both litter quality and incubation site on litter decomposition and N release. Moreover, previous studies on the QTP investigating how grazing exclusion influences plant abundance and biodiversity usually lasted for many years, and the short-term effects have rarely been reported. This work studied the short-term (6 months) effects of grazing and grazing exclusion on plant community composition (i.e., plant species presented) and litter quality and long-term (27–33 months) effects on soil chemical characteristics and mixed litter decomposition and N release on the QTP. Our results demonstrate that (1) shorter-term grazing exclusion had no effect on plant community composition but increased plant palatability and total litter biomass; (2) grazing resulted in higher N and C content in litter; and (3) grazing accelerated litter decomposition, while grazing exclusion promoted N release from litter and increased soil organic carbon. In addition, incubation site had significantly more impact than litter quality on litter decomposition and N release, while litter quality affected decomposition in the early stages. This study provides insights into the mechanisms behind the nutrient cycling in alpine ecosystems. We suggest that periodic grazing and grazing exclusion is beneficial in grassland management on the QTP.
- Article
(572 KB) -
Supplement
(418 KB) - BibTeX
- EndNote
The Qinghai-Tibetan Plateau (QTP) represents an important ecoregion in China (Wen et al., 2010), in which alpine grasslands cover more than 85 % of total area and are regarded as the major land unit of natural pastures in China (Dong et al., 2010). However, the grassland systems in this region have suffered from severe degradation driven by a range of factors including climate change, overgrazing, overcultivation and poor management (Han et al., 2008; Li et al., 2009; Wu et al., 2009, 2010; Feng et al., 2010), and the degraded land area has been increasing at 1.2–7.44 % per year (Ma et al., 2007). Since the 1990s, the restoration of degraded grasslands has attracted considerable attention (Kang et al., 2007; Han et al., 2008), and some efforts have recently been directed towards grassland restoration and maintenance by increasing aboveground plant abundance (Niu et al., 2009) and biodiversity (Wu et al., 2009; Niu et al., 2010) and improving soil organic matter content and nutrient availability (Cao et al., 2004; Wu et al., 2010; Sun et al., 2011). It is well known that grazing may change the vegetation community structure, soil structure and nutrient cycling processes, and that such changes have important consequential impacts on the structure and functioning of the ecosystem as a whole. However, litter decomposition and N release, the key factors regulating the nutrient cycle and availability at the soil–plant interface through grazing (Carrera and Bertiller, 2013), are as yet little studied in these alpine ecosystems (Luo et al., 2010; Zhu et al., 2016).
Herbivore grazing may induce short-term ecophysiological changes in plant tissues, which in turn may translate into litter quality changes and long-term shifts in plant community composition. At the short-term ecophysiological level, grazing may promote the plant species producing high-quality litter (Holland and Detling, 1990; Sirotnak and Huntly, 2000; Olofsson and Oksanen, 2002; Semmartin et al., 2004, 2008) because the consuming of plant tissues by herbivores may favor the grazed species with a higher re-growth rate and greater nutrient contents in plant tissues due to the higher nutrient uptake (see Holland and Detling, 1990; Olofsson and Oksanen, 2002; Semmartin et al., 2008). At the long-term community level, selective foliar grazing may alter the competitive interactions and recruitment patterns of plant species, which may change their abundance and life-form structure (Bardgett and Wardle, 2003; Semmartin et al., 2008; Wu et al., 2009; Niu et al., 2010). For instance, herbivores preferentially feed on the most palatable plants (e.g., species with high nutrient and low fibre contents), which may promote dominance of unpalatable species (Garibaldi et al., 2007), resulting in the high inputs of low-quality litter to soil and thus a reduction of decomposition rate, nutrient availability and nutrient cycling (Ritchie and Knops, 1998; Moretto et al., 2001; Olofsson and Oksanen, 2002). Therefore, litter in grassland subject to long-term grazing may decompose more slowly. However, some studies demonstrate that grazing per se may accelerate litter decomposition by modifying site conditions for litter turnover through physical changes in the soil by herbivore activities, such as trampling and urine/dung deposition (Takar et al., 1990; Fahnestock and Knapp, 1994; Semmartin et al., 2008; Luo et al., 2010; Liang et al., 2018). Empirical evidence of variance in litter quality input and decomposition caused by grazing is still subject to debate (Garibaldi et al., 2007).
It is often assumed that higher nutrient content in plant tissue usually results in faster litter decomposition and in higher nutrient mineralization and availability in soil (Olofsson and Oksanen, 2002). At the ecosystem scale, the chemical characteristics of plant litter, for example the carbon : nitrogen ratio (C : N) and/or nitrogen and lignin content, are often regarded as the indicators of litter quality (Aerts, 1997; Strickland et al., 2009). Many studies have demonstrated a positive correlation between litter decomposition rate and litter N content, or a negative relationship between litter decomposition rate and initial litter lignin content and C : N or lignin : N ratio (e.g., Wardle et al., 2002; Aerts et al., 2003; Semmartin et al., 2004; Garibaldi et al., 2007; Luo et al., 2010; Vaieretti et al., 2013).
In addition to litter quality (its chemical composition), two further factors controlling litter decomposition are the climate (mainly temperature and humidity) and decomposing organisms (their abundance and activity) (Coûteaux et al., 1995; Aerts, 1997; Semmartin et al., 2004; Keeler et al., 2009; Berg and McClaugherty, 2014; Zhu et al., 2016). Climate usually regulates decomposition processes at global and regional scales (Coûteaux et al., 1995; Silver and Ryan, 2001), but microbial activity regulates decomposition processes through soil temperature and moisture effects (modified by grazing) at a local scale (Coûteaux et al., 1995; De Santo et al., 1993; Luo et al., 2010; Orsborne and Macauley, 1988). Generally, climatic influence dominates litter quality and decomposer activity in areas where weather conditions are unfavorable (Coûteaux et al., 1995), due to the dependence of decomposer activity on microclimate (De Santo et al., 1993). Under favorable conditions, litter quality may largely prevail as the regulator and remain important until the late decomposition stages (Coûteaux et al., 1995). However, specific temperature and moisture conditions and litter quality may interact strongly and thus the rate of litter decomposition is difficult to predict.
Most studies evaluating the effect of grazing on litter decomposition usually focus on forest, grassland or crop ecosystems in temperate areas (e.g., Aber and Melillo, 1980; Berg and Staaf, 1981; Luo et al., 2010; McCurdy et al., 2013), largely ignoring those in the alpine zones. On the QTP, previous studies prove that long-term grazing exclusion (> 2 years) may promote plant abundance and biodiversity (Niu et al., 2009, 2010; Wu et al., 2009, 2010); however, exclusion may limit the efficient use of grassland. By contrast, the short-term effect of grazing exclusion is seldom studied. Other previous studies have focused on the dominant species only (e.g., Wu et al., 2009; Luo et al., 2010) and this approach provides less insight into the nature of nutrient cycling in the grasslands than work on mixed litter (Zhu et al., 2016; this study). Moreover, few studies have simultaneously investigated how both litter quality and incubation site affect litter decomposition (e.g., Luo et al., 2010; Zhu et al., 2016; Liang et al., 2018). In this study, we examined the short-term effect (6 months) of grazing and grazing exclusion on plant community composition and litter quality and their longer-term effect on mixed litter decomposition and N release (27 months) and soil chemical characteristics (33 months). Based on the information above, this research aims to test three hypotheses: (1) short-term grazing exclusion does not change plant community composition and litter quality (i.e., nutrient content as N and biomass of palatable plant species), (2) grazing may accelerate litter decomposition and N release and thus increase soil organic carbon and N, and (3) litter quality has less effect on litter decomposition and N release compared to incubation site. Results of the present study may improve our understanding of nutrient cycling in alpine regions in general, and this study may also further provide knowledge relevant to the development of strategies for restoring the degraded grasslands on the QTP in particular.
2.1 Experimental site
This study site was an alpine meadow on the eastern QTP, SW China (33∘59′ N, 102∘00′ E, altitude 3500 m above sea level). The mean annual temperature is 1.2 ∘C, ranging from −10 ∘C in January to 11.7 ∘C in July, with approximately 270 days with frost per year. The mean annual precipitation over the last 35 years, occurring mainly during the short and cool summer is 620 mm (Niu et al., 2010). The years during this study (i.e., 2009–2012) were climatically typical (Sun et al., 2015; Supplement Fig. S1).
The grassland selected for experiments was > 9 ha in area (including 6 ha of experimental plots and 3 ha of buffer areas) and regularly used for Tibetan sheep and yak grazing during the grazing season (May–October). The slopes at the site are less than 5 %, typical of the gently undulating topography of the region. The soil properties in the experimental plots were similar after a long-term grazing history with the same grazing pattern. The soil type at the experiment site is an alpine meadow soil, similar to the Mat-Cryic Cambisols described by Wu et al. (2010).
2.2 Litter composition and quality
To measure the annual litter composition and determine whether plants could recover without grazing, three grazing (GP, 100 m × 200 m) and three grazing exclusion paddocks (GEP, 30 m × 20 m) were established when all aboveground plants were dormant in October 2009. Grazing in GP started with an optimal moderate stocking rate of 4 Tibetan sheep ha−1 from April 2010. The mean body weight of sheep was about 38 kg when used for the experiment. Before grazing started, 20 quadrats (0.5 m × 0.5 m) identified by GPS coordinates were randomly established within the GP or GEP, and litter was cleared soon after the establishment of quadrats. In October 2010, we collected all plant litter from each quadrat of the GP and GEP for two purposes: (1) measurement of litter composition and quality in this experiment, and (2) measurement of litter decomposition and N release in the next experiment. Three sampling methods were designed to minimize the sample variance caused by the uneven litter distribution and to ensure the similar composition and quality of litter used for this and the next experiment: (1) half alongside, (2) half along diagonal and (3) two sub-quarters (0.25 × 0.25 m) along diagonal (Supplement Fig. S2).
To measure the litter composition, litter of different species collected from each quarter was identified. After litter species identification, litter was separated into two groups of contrasting palatability to the Tibetan sheep (Niu et al., 2009, 2010; Wu et al., 2009; see Supplement Table S1): (1) palatable species – preferred and desirable species, and (2) unpalatable species – undesirable and toxic species. To measure the dry biomass, the palatable and unpalatable litter was separately oven-dried at 60 ∘C for 48 h and then weighed.
To measure the quality of litter collected from GP (GP-litter treatment) or from GEP (GEP-litter treatment), the palatable and unpalatable litter from a quarter was mixed again, then ground and stored in a ziplock bag with 10 g per bag for a quality test. There were six replicates for each treatment. The contents of lignin, cellulose and hemicellulose were measured as described by van Soest et al. (1991). Organic carbon concentration (C) was measured by the K2Cr2O7-H2SO4 oxidation method of Walkley–Black (Nelson and Sommers, 1996). The total Kjeldahl nitrogen (N) and total phosphorus (P) were analyzed using a FIAstar 5000 flow injection analyzer (FOSS Tecator, Högnäs, Sweden) (Chen et al., 2016). We also calculated the ratios of C : N, lignin : N, cellulose : N and hemicellulose : N.
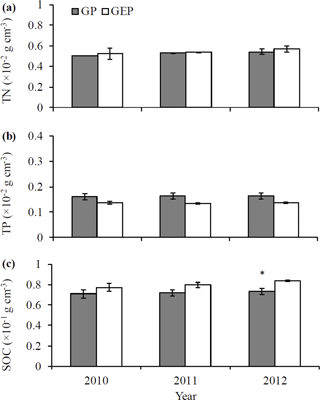
We also examined the effects of grazing and grazing exclusion on soil characteristics. We randomly collected five soil samples in each experimental paddock (n=30 in total) from the 0–10 cm depth using a bucket auger (10 cm in diameter) in October 2010, 2011 and 2012. The same methods used to test litter quality (i.e., Nelson and Sommers, 1996; Chen et al., 2016) were applied to estimate the soil organic carbon (SOC), total nitrogen (TN) and total phosphorus (TP).
2.3 Litter decomposition and N release
In this experiment, we included four treatments: (1) GP-GP, litter of all species was collected from and incubated in the GP; (2) GEP-GEP, litter of all species was collected from and incubated in the GEP; (3) GP-GEP, litter of all species was collected from the GP but incubated in the GEP; (4) GEP-GP, litter of all species was collected from the GEP but incubated in the GP. Treatments 1 and 2 were designated “in situ” incubation treatments, while treatments 3 and 4 were designated “across” grazing category incubation treatments, and these were included to improve understanding of the “home-field advantage” effect on litter deposition (John et al., 2011).
For each sample soil particles attached to the litter were cleaned off with a soft brush, and samples were air-dried for 3 days. Dry litter collected from each quadrat was cut to ≈ 5 cm length and 10 g litter was packed into a nylon litter bag (15 cm × 20 cm with mesh size of 0.35 mm) (Cornelissen, 1996), which should have prevented any loss of material and had no effect on litter decomposition (Cornelissen et al., 1999). On 20 October 2010, the packed litter was incubated above the soil surface by fastening to the ground surface with four steel stakes to prevent removal by sheep and small animals (Vaieretti et al., 2013), such as the plateau pika Ochotona curzoniae (Hodgson). For each treatment, 24 litter bags were incubated 20 cm apart from each other to reduce the mutual interference. Three litter bags from each treatment were retrieved after incubation periods of 56, 141, 247, 391, 444, 582, 695 or 799 days (i.e., on 15 December 2010, 10 March, 24 June and 4 September 2011, and 7 January, 24 May, 14 September and 27 December 2012, respectively). There were a total of 96 litter bags used in this experiment. Retrieved litter was brought back to the laboratory, cleaned by removing any extraneous material attached and then weighed after being oven-dried at 60 ∘C for 48 h. Samples were ground and stored in a ziplock bag for further chemical analyses as mentioned above. We estimated the litter decomposition and N release as the percentage of dry weight lost during each incubation period (Cornelissen et al., 1999; Vaieretti et al., 2013).
Table 1Initial chemical characteristics (mean ± SE) of litter collected from grazing paddocks (GP litter) and grazing exclusion paddocks (GEP litter). Unit of chemical characteristics is milligrams per gram of litter for C, N, P, lignin, cellulose and hemicellulose. Different letters in each row indicate significant difference (ANOVA: P<0.05).
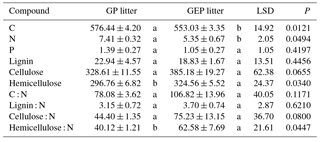
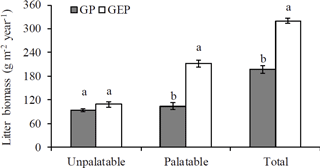
Figure 1Mean (± SE) annual biomass of litter collected from grazing paddocks (GP) and grazing exclusion paddocks (GEP). For each category, columns with different letters are significantly different (ANOVA: P<0.05).
2.4 Statistical analyses
A goodness-of-fit test (Shapiro–Wilk test, univariate procedure) was used to test the normality of data before mean comparison using analysis of variance (ANOVA, GLM procedure). All data were normally distributed. Data on the initial chemical characteristics of litter (Table 1) were analyzed using ANOVA followed by Tukey's Studentized multiple range test. Data on the biomass of palatable or unpalatable species and those on the total biomass between GP and GEP were also analyzed using ANOVA, while for GP or GEP the difference in litter biomass between the palatable and unpalatable species was compared using a paired-t test (TTEST procedure) (Fig. 1). Data on the final proportion of litter biomass or N remaining (Fig. 4), litter quality (content of organic carbon, nitrogen, phosphorous and other chemical characteristics of litter) (Table 1), and soil SOC, TN and TP (Fig. 2) were analyzed using ANOVA followed by the least significant difference test (LSD test) for multiple comparisons.
Table 2Litter decay rate (k, ) in different incubation environments. GP-GP, mixed litter was collected from grazing paddocks (GP) and incubated in GP; GEP-GEP, mixed litter was collected from grazing exclusion paddocks (GEP) and incubated in GEP; GEP-GP, mixed litter was collected from GEP and incubated in GP; GP-GEP, mixed litter was collected from GP and incubated in GEP. k values followed by different letters are significantly different (non-overlap of 83.4 % CL). R2, F and P are estimated from the negative exponential model of Swift et al. (1979).

The decomposition rate (k, g 10 ) of litter biomass during the incubation period (Table 2) was assessed using a negative exponential model according to Swift et al. (1979): , where y is the dry biomass of litter remaining in the litter bags at time t (days), and a is the initial litter biomass (i.e., 10 g in this study). The difference in decomposition rate between treatments was compared according to Julious (2004); i.e., there is deemed to be no significant difference in decomposition rate if there is a 83.4 % CL (confidence limit) overlap. The decomposition rate and 83.4 % CL were estimated by fitting the negative exponential model to a nonlinear least-square regression model (NLIN procedure).
Table 3Contribution (%) of incubation site (site: GP, grazing paddocks; GEP, grazing exclusion paddocks) and litter quality (quality: GP litter, mixed litter collected from grazing paddocks; GEP litter, mixed litter collected from grazing exclusion paddocks) to litter decomposition and N release.
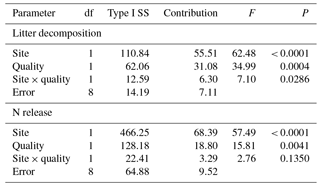
A multivariate regression model (GLM procedure) employed by Vaieretti et al. (2013) was applied to quantify the effect of incubation site and litter quality (the two independent factors) on the final litter decomposition or N release (the dependent factor) (Table 3): litter decomposition or N release = site + quality + site × quality + ϵ, where “site” is the paddock category in which the litter was incubated (i.e., incubation site: GP and GEP), “quality” is the litter quality reflecting the sources from which the litter was collected (i.e., GP and GEP) and ϵ is the model error. The proportional contribution of incubation site, litter quality and their interaction to variability in litter decomposition or N release was calculated as the sum of squares for each of the terms, divided by the total sum of squares. The Type I sum of squares was used because of the balanced design of this experiment. All analyses were carried out using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). The rejection level for H0 was set at α<0.05. Values of the means (±SE) are presented in Figs. 1–4.
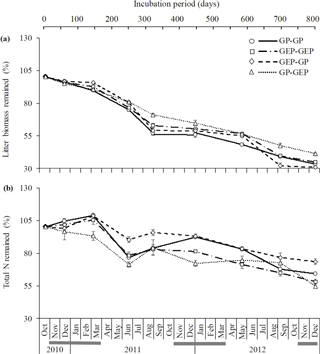
Figure 3Dynamics (mean ± SE) of litter decomposition (a) and N lease (b) on the QTP. GP-GP: mixed litter was collected from grazing paddocks (GP) and incubated in GP; GEP-GEP: mixed litter was collected from grazing exclusion paddocks (GEP) and incubated in GEP; GEP-GP: mixed litter was collected from GEP and incubated in GP; GP-GEP: mixed litter was collected from GP and incubated in GEP. Grey lines under months indicate the mean air temperatures < 0 ∘C.
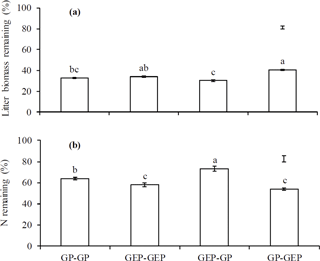
Figure 4Percentage of litter mass (a) and total N remaining (b) at the end of experiments. GP-GP: mixed litter was collected from grazing paddocks (GP) and incubated in GP; GEP-GEP: mixed litter was collected from grazing exclusion paddocks (GEP) and incubated in GEP; GEP-GP: mixed litter was collected from GEP and incubated in GP; GP-GEP: mixed litter was collected from GP and incubated in GEP. Vertical bars are the least significant difference (LSD) values. Columns with the different letters are significantly different (LSD test: P<0.05).
3.1 Litter composition and quality and soil property
There were 55 plant species (mostly forbs and grasses along with several legumes and sedges) identified, and all were found in both GP and GEP, except Gentiana macrophylla Pallas, which was only found in the GP (Supplement Table S1). However, even though the annual litter biomass of unpalatable species in both GP and GEP was similar (ANOVA: F1.38=3.43, P=0.0717), litter biomass of palatable species was significantly greater in the GEP than in the GP (ANOVA: F1.38=75.32, P<0.0001), and this difference contributed significantly more to the total litter biomass in the GEP than in the GP (ANOVA: F1.38=114.66, P<0.0001) (Fig. 1). The litter biomass was not significantly different between palatable and unpalatable species in the GP (paired-t test: t19=0.96, P=0.3510); however, in the GEP, litter biomass of palatable species was significantly greater than that of unpalatable species (paired-t test: t19=7.17, P<0.0001) (Fig. 1).
GP litter had significantly higher C and N but significantly lower hemicellulose and hemicellulose : N than GEP litter (Table 1). Although other quality characteristics were lower in GP litter than in GEP litter, the differences were not significant (Table 1).
The concentrations of soil TN and TP were not significantly different between the GP and GE for any year (LSD = 0.0002–0.0015 and 0.0003–0.0004 for TN and TP, respectively; P>0.05) (Fig. 2a–b). Similarly, there was no significant difference in SOC concentration between the GP and GE in 2010 and 2011 (LSD = 0.0169 and 0.0111 for 2010 and 2011, respectively; P>0.05), while in 2012 SOC was significantly higher in GEP than in GP (LSD = 0.0138, P=0.0279) (Fig. 2c).
3.2 Litter decomposition
The proportion of litter biomass remaining continuously decreased with incubation duration, and litter decomposed faster in the first year (i.e., 44.6, 38.5, 41.46 and 31.6 % decomposition in GP-GP, GEP-GEP, GEP-GP and GP-GEP, respectively) than in the second year (i.e., 18.8, 24.1, 27.6 and 23.4 % decomposition in GP-GP, GEP-GEP, GEP-GP and GP-GEP, respectively) (Fig. 3a). As shown in Table 2, the decomposition rate (k) of litter incubated in GP was significantly higher than that in GEP (non-overlap of 83.4 % CL), i.e., for the in situ treatments k in GP-GP > k in GEP-GEP and for the across treatments k in GEP-GP > k GP-GEP. The final proportion of litter biomass remaining was significantly lower in GP-GP and GEP-GP than in GP-GEP (LSD = 2.51, P<0.0001) (Fig. 4a).
3.3 N release
Generally, the percentage of total N release did not change during the first winter when temperature was < 0 ∘C, except that it increased during the incubation period of December 2010 and March 2011 (first winter) in the treatment GEP-GP (Fig. 3b). From January in the second winter (2012), the percentage of total N remaining decreased steadily until the end of the experiment (Fig. 3b). The final proportion of total N remaining was significantly higher in GEP-GP and significantly lower in GEP-GEP and GP-GEP (LSD test: LSD = 5.36, P=0.0002) (Fig. 4b).
3.4 Contribution of incubation site and litter quality to litter decomposition and N release
The multivariate regression model indicates that both incubation site and litter quality significantly affected litter decomposition and N release (Table 3). Incubation site contributed respectively near 25 and 50 % more to litter decomposition and N release than litter quality (Table 3). Furthermore, the model predicts that GP resulted in significantly greater litter decomposition (8.13 %) but significantly lower N release (9.73 %) than did GEP (F1.8=62.48 and 57.49 for litter decomposition and N release, respectively; P<0.0001). Results show that GEP litter decomposed significantly faster (2.5 %) but released N significantly more slowly (9.27 %) than GP litter (F1.8=34.99 and 15.80 for litter decomposition and N release, respectively; P<0.01).
A significant interaction between incubation site and litter quality for litter decomposition was found (F2.8=7.10, P=0.0286); i.e., litter collected from GEP but incubated in GP decomposed significantly faster (also see Fig. 4a). However, the interaction between incubation site and litter quality on N release was not significant (F2.8=2.76, P=0.1350).
4.1 Litter composition and quality
Grazing or grazing exclusion of herbivores may indirectly alter the species composition and functioning of grasslands by inducing shifts in plant competitive interactions and recruitment patterns and thus changes in species abundance and life-form structure (Bardgett and Wardle, 2003; Garibaldi et al., 2007; Semmartin et al., 2008; Wu et al., 2009; Niu et al., 2010; Chaneton, 2011). However, our results indicate that herbivore grazing or grazing exclusion did not alter plant community composition in terms of species inventory, as species found in the GEP mostly also occurred in the GP. On the QTP, species composition is grazing intensity dependent (Niu et al., 2010; Sun et al., 2011) and/or grazing exclusion period dependant (Wu et al., 2009). Thus our results imply that a stocking rate of 4 Tibetan sheep ha−1 in the GP or a short period of grazing exclusion (i.e., from April to October) in the GEP did not change the species composition.
However, our results show that herbivore grazing significantly altered species composition in terms of species abundance or palatability, with significantly less palatable as well as total litter produced in the GP compared to the GEP (Fig. 1). The low biomass of palatable species in GP may be attributed to the more palatable species (mostly the grasses and sedges; see Supplement Table S1) on the QTP being taller (Sun et al., 2011), and therefore more accessible to herbivore grazing, in addition to being more likely to be grazed by preferential grazing. Through these two mechanisms, the biomass of palatable species in the GP would subsequently be reduced. Results of this study indicate that grazing exclusion for a short period may allow the recovery of palatable species in the alpine meadows. However, there was no significant difference in the litter biomass of unpalatable species between the GP and GEP (Fig. 1), which provides evidence against the assumption that removing the canopy of palatable species may allow intra- and inter-specific competition for light, which ultimately favors the establishment of short, less-palatable species (Sternberg et al., 2000; Pavlů et al., 2008; Wu et al., 2009; Sun et al., 2011).
It is generally accepted that litter quality is usually determined by the levels of various chemical compounds such as soluble C, N and P, as well as lignin or lignin : N ratio, and litter of high quality usually has higher N content but lower lignin and lignin : N ratio (e.g., Aerts, 1997; Olofsson and Oksanen, 2002; Wardle et al., 2002; Garibaldi et al., 2007; Strickland et al., 2009). In this study, grazing might have improved litter quality at least to some extent by significantly increasing the N content and potentially reducing the hemicellulose content and C : N, lignin : N, cellulose : N and hemicellulose : N ratios (Table 1). Therefore, our results align with previous studies that have indicated grazing may promote litter quality due to the high nutrient uptake (e.g., Sirotnak and Huntly, 2000; Olofsson and Oksanen, 2002; Semmartin et al., 2004).
4.2 Litter decomposition
For a given climatic region, the ecological processes of litter decomposition are regulated by incubation microenvironment (i.e., grazing and grazing exclusion and soil property in this study) and litter quality. Our results suggest that herbivore grazing played a major role in litter decomposition on the QTP. Many studies have demonstrated that litter quality is one of the most important factors affecting the litter decomposition, and litter with higher N content but lower lignin and lignin : N ratio will decompose faster (Aerts, 1997; Olofsson and Oksanen, 2002; Wardle et al., 2002; Garibaldi et al., 2007; Strickland et al., 2009). Therefore, it may be expected that regardless of incubation site, GP litter that had significantly higher N content should decompose faster than GEP litter. However, evidence for this expectation was only detected for the in situ incubation treatments (i.e., greater decomposition rate in GP-GP than in GEP-GEP), while for the across incubation treatments opposite results were found (i.e., greater decomposition rate in GEP-GP than in GP-GEP) (Table 2 and Fig. 4a). Our experimental site is located at high altitude with a low mean annual temperature of 1.2 ∘C; hence the activity of decomposers may be inhibited during the cold seasons (Coûteaux et al., 1995). Therefore, litter quality may not be a good predictor of litter decomposability in cold temperate regions (Aerts, 1997), where climate is more important than litter quality in the regulation of litter decomposition (Coûteaux et al., 1995). On the QTP, decomposition rate (k=1.04 × 10−3–1.34 × 10−3 in Table 2, i.e., k=0.38–0.48 ) was much lower than that of the global mean (k≈0.75 ) with similar latitudes (30–40∘ N) (Zhang et al., 2008).
Additionally, incubation site had a significantly greater effect on litter decomposition (≈ 25 %) than litter quality (Table 3), and regardless of litter quality, litter decomposed faster in GP (19.9 % for GP litter and 11.8 % for GEP litter) than in GEP (Fig. 4a; Table 2). This may be attributed to the effect of herbivore grazing activity, which modifies the incubation site conditions for litter turnover (Takar et al., 1990; Fahnestock and Knapp, 1994; Semmartin et al., 2008; Luo et al., 2010; Liang et al., 2018). Our results further demonstrate that regardless of incubation site and litter quality, litter decomposed faster in the first year (31.6–44.6 % decomposition) than in the second year (18.8–27.6 % decomposition) (Fig. 3a). Berg (2014) and Berg and McClaugherty (2014) have stated that litter decomposition rate varies at different stages. In the early stages of decomposition litter mass is lost rapidly via the leaching of soluble compounds, while in the later stages decomposition can even cease after only recalcitrant litter compounds remain (Berg, 2014; Berg and McClaugherty, 2014). Therefore, litter quality regulates decomposition processes mainly in the early stages of decomposition (Berg and McClaugherty, 2014), which is supported by our results, i.e., when incubated in GP in the first year, the percentage decomposition of GP litter (44.6 %) was higher than that of GEP litter (41.5 %). However, we could not reject the above conclusion that incubation site is the dominant factor affecting litter decomposition over litter quality, as in the first year litter always decomposed faster in GP (i.e., 44.6 % in GP-GP and 41.5 % in GEP-GP) than in GEP (i.e., 38.5 % in GEP-GEP and 31.6 % in GP-GEP) (Fig. 3a). These data also imply that a home-field advantage is detected only for GP litter during the first year of incubation (i.e., percentage decomposition of 44.6 % in GP-GP > 31.6 % in GP-GEP) with no evidence for GEP litter (i.e., percentage decomposition of 38.5 % in GEP-GEP < 41.5 % in GEP-GP).
In a long-term (9 years) study on the QTP, Wu et al. (2009) reported that grazing exclusion favors the increase in soil TN, soil organic matter, SOC, soil microbial biomass carbon and soil carbon storage. It is interesting that in the present study, only SOC significantly increased after 3 years of grazing exclusion (Fig. 2). The increase in SOC in GEP may be because grazing exclusion prevents the reduction of removal of palatable litter by the herbivores (Fig. 1), and the organic C locked within plant tissues may be returned to the soil during litter decomposition instead (Bardgett and Wardle, 2003; Wu et al., 2009). Holland and Detling (1990) and Ågren et al. (1999) stated that increasing carbon availability in soil may promote decomposer growth and activity even at low nitrogen concentrations. However, the expected results, i.e., significantly higher litter decomposition rate caused by the possible increasing decomposer mass and/or activity in the grazing exclusion grasslands (Wu et al., 2009), were not observed in the GEP in this study (Table 2, Fig. 4a). Thus, soil properties are unlikely to be significantly changed through grazing or grazing exclusion over relatively short periods, indicating that limited grazing events have a smaller effect on litter decomposition under cool environments on the QTP than in experiments conducted in warmer climates.
4.3 N release
N release is a more complex process compared to litter decomposition. N release may involve any one or both processes of N immobilization and N mineralization, where the former results in the accumulation of N in the litter and the latter causes the release of N from the litter (Manzoni et al., 2008). Swift et al. (1979) and Berg and McClaugherty (2014) reported that the biological decomposition of litter is mainly carried out by microbial decomposers, which per se have a higher N : C ratio compared with most litter types. This property of decomposers creates a high N demand for decomposer growth (Manzoni et al., 2008). Results show that regardless of litter quality or source, N remaining was significantly higher when litter was incubated in GP than in GEP (Fig. 4b). Bosatta and Balesdent (1996) and Manzoni et al. (2008) show that a promising candidate mechanism may be that the faster decomposition rate of litter in GP increases the utilization of C by the decomposers, which in turn increases the N : C ratio in litter; when N : C ratio is high, large amounts of mineral N are immobilized, increasing the N concentration in litter. This mechanism may also account for the dynamics of N release over the incubation period (Fig. 3b). For instance, decomposer activity resulted in continuous C consumption and litter decomposition (March–June 2011), while high N : C ratio due to decomposition progress induced the release of accumulated N (June–August 2011). The inverse pattern of N release and litter decomposition found in this study (Fig. 4) is frequently reported (e.g., Aber and Melillo, 1980; Fahey et al., 1991; Gallardo and Merino, 1992).
It is not surprising that because both litter decomposition and N release are regulated by decomposers synchronously, incubation site also had a significantly greater effect (≈ 50 %) on N release on litter decomposition (Table 3) than litter quality, indicating the latter is not a good predictor on litter N release in the cold temperate region (Aerts, 1997).
Results of our study are not completely consistent with previously proposed hypotheses. On the cold QTP, short-term grazing exclusion did not promote species abundance but increased plant palatability and total litter biomass. Grazing improves litter quality through higher N content but lower hemicellulose and hemicellulose : N ratio. Grazing significantly accelerated litter decomposition, while grazing exclusion promoted N release and increased SOC. Although litter quality may affect decomposition in the early stages, incubation site had significantly more of an impact on both litter decomposition and N release. The different effects of grazing and grazing exclusion functioning on the grassland ecosystems may have implications in the management of alpine meadows on the QTP. For example, periodic grazing and grazing exclusion may be good options that allow plant recovery and promote nutrient cycling, and thus contribute to the restoration of degraded grasslands.
Data are available from the author, Fujiang Hou (cyhoufj@lzu.edu.cn), upon request.
The supplement related to this article is available online at: https://doi.org/10.5194/bg-15-4233-2018-supplement.
YS and FH designed and FH supervised the experiments. YS, FH, ZW and SC performed research and collected data. XH, FH and YS analyzed data and prepared the manuscript, and all authors contributed to the writing.
The authors declare that they have no conflict of interest.
We are very grateful to Jens-Arne Subke (editor) and two anonymous reviewers
for their constructive comments and suggestions, which have significantly
improved the paper. We are also grateful to Cory Matthew for his
valuable comments and time spent editing the English of a previous version of the paper. This
study was financially supported by the National Key Project of Scientific
and Technical Supporting Programs (2014CB138706), National Natural Science
Foundation of China (no. 31672472), Program for Changjiang Scholars and
Innovative Research Team in University (IRT_17R50) and the independent grants
from the State Key Laboratory of Grassland Agro-ecosystems (SKLGAE201708).
Edited by: Jens-Arne Subke
Reviewed by: two anonymous referees
Aber, J. D. and Melillo, J. M.: Litter decomposition: measuring relative contributions of organic matter and nitrogen to forest soils, Can. J. Biochem., 58, 416–421, https://doi.org/10.1139/b80-046, 1980.
Aerts, R.: Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship, Oikos, 79, 439–449, https://doi.org/10.2307/3546886, 1997.
Aerts, R., De Caluwe, H., and Beltman, B.: Plant community mediated vs. nutritional controls on litter decomposition rates in grasslands, Ecology, 84, 3198–3208, https://doi.org/10.1890/02-0712, 2003.
Ågren, G. I., Shaver, G. R., and Rastetter, E. B.: Nutrients: dynamics and limitations, in: Carbon dioxide and environmental stress, edited by: Luo, Y. and Mooney, H. A., Academic Press, San Diego, 333–345, 1999.
Bardgett, R. D. and Wardle, D. A.: Herbivore-mediated linkages between aboveground and belowground communities, Ecology, 84, 2258–2268, https://doi.org/10.1890/02-0274, 2003.
Berg, B.: Decomposition patterns for foliar litter – A theory for influencing factors, Soil Biol. Biochem., 78, 222–232, https://doi.org/10.1016/j.soilbio.2014.08.005, 2014.
Berg, B. and McClaugherty, C.: Plant litter: decomposition, humus formation, carbon sequestration, 3rd edn., Springer, 315 pp., 2014.
Berg, B. and Staaf, H.: Leaching, accumulation and release of nitrogen in decomposing forest litter, in: Terrestrial Nitrogen Cycles, edited by: Clark, F. E. and Rosswall, T., Processes, Ecosystem Strategies and Management Impacts, Ecological Bulletins, Stockholm, Sweden, 33, 163–178, 1981.
Bosatta, E. and Balesdent, J.: Isotope discrimination during decomposition of organic matter: a theoretical analysis, Soil Sci. Soc. Am. J., 60, 1121–1126, https://doi.org/10.2136/sssaj1996.03615995006000040023x, 1996.
Cao, G. M., Tang, Y. H., Mo, W. H., Wang, Y. S., Li, Y. N., and Zhao, X. Q.: Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau, Soil Biol. Biochem., 36, 237–243, https://doi.org/10.1016/j.soilbio.2003.09.010, 2004.
Carrera, A. L. and Bertiller, M. B.: Combined effects of leaf litter and soil microsite on decomposition process in arid rangelands, J. Environ. Manage., 114, 505–511, https://doi.org/10.1016/j.jenvman.2012.10.059, 2013.
Chaneton, E. J.: Linking grazing-induced changes in plant biodiversity to rangeland ecosystem function, IX International Rangeland Congress, Lugar, Rosario, Año, 286–291, 2011.
Chen, X. J., Hou, F. J., Matthew, C., and He, X. Z.: Soil C, N, and P stocks evaluation under major land uses on China's Loess Plateau, Rangeland Ecol. Manage., 70, 341–347, https://doi.org/10.1016/j.rama.2016.10.005, 2016.
Cornelissen, J. H. C.: An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types, J. Ecol., 84, 573–582, https://doi.org/10.2307/2261479, 1996.
Cornelissen, J. H. C., Harguindeguy, N. P., Díaz, S., Grime, J. P., Marzano, B., Cabido, M., Vendramini, F., and Cerabolini, B.: Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents, New Phytol., 143, 191–200, https://doi.org/10.1046/j.1469-8137.1999.00430.x, 1999.
Coûteaux, M. M., Bottner, P., and Berg, B.: Litter decomposition, climate and litter quality, Trends Ecol. Evol., 10, 63–66, https://doi.org/10.1016/S0169-5347(00)88978-8, 1995.
De Santo, A. V., Berg, B., Rutigliano, F. A., Alfani, A., and Floretto, A.: Factors regulating early-stage decomposition of needle litters in five different coniferous forests, Soil Biol. Biochem., 25, 1423–1433, https://doi.org/10.1016/0038-0717(93)90057-I, 1993.
Dong, S. K., Wen, L. U., Zhu, L., and Li, X. Y.: Implication of coupled natural and human systems in sustainable rangeland ecosystem management in HKH region, Front. Earth Sci. China, 4, 42–50, https://doi.org/10.1007/s11707-010-0010-z, 2010.
Fahey, T. J., Stevens, P. A., Hornung, M., and Rowland, P.: Decomposition and nutrient release from logging residue following conventional harvest of Sitka spruce in North Wales, Forestry, 64, 289–301, https://doi.org/10.1093/forestry/64.3.289, 1991.
Fahnestock, J. T. and Knapp, A. K.: Plant responses to selective grazing by bison: interactions between light, herbivory and water stress, Vegetatio, 115, 123–131, https://doi.org/10.1007/BF00044867, 1994.
Feng, R. Z., Long, R. J., Shang, Z. H., Ma, Y. S., Dong, S. K., and Wang, Y. L.: Establishment of Elymus natans improves soil quality of a heavily degraded alpine meadow in Qinghai-Tibetan Plateau, China, Plant Soil, 327, 403–411, https://doi.org/10.1007/s11104-009-0065-3, 2010.
Gallardo, A. and Merino, J.: Nitrogen immobilization in leaf litter at two Mediterranean ecosystems of SW Spain, Biogeochemistry, 15, 213–228, https://doi.org/10.1007/BF00002937, 1992.
Garibaldi, L. A., Semmartin, M., and Chaneton, E. J.: Grazing-induced changes in plant composition affect litter quality and nutrient cycling in flooding Pampa grasslands, Oecologia, 151, 650–662, https://doi.org/10.1007/s00442-006-0615-9, 2007.
Han, J. G., Zhang, Y. J., Wang, C. J., Bai, W. M., Wang, Y. R., Han, G. D., and Li, L. H.: Rangeland degradation and restoration management in China, Rangeland J., 30, 233–239, https://doi.org/10.1071/RJ08009, 2008.
Holland, E. A. and Detling, J. K.: Plant response to herbivory and belowground nitrogen cycling, Ecology, 71, 1040–1049, https://doi.org/10.2307/1937372, 1990.
John, M. G. S., Orwin, K. H., and Dickie, I. A.: No `home' versus `away' effects of decomposition found in a grassland–forest reciprocal litter transplant study, Soil Biol. Biochem., 43, 1482–1489, https://doi.org/10.1016/j.soilbio.2011.03.022, 2011.
Julious, S. A.: Using confidence intervals around individual means to assess statistical significance between two means, Pharm. Stat., 3, 217–222, https://doi.org/10.1002/pst.126, 2004.
Kang, L., Han, X. G., Zhang, Z. B., and Sun, O. J. X.: Grassland ecosystems in China: review of current knowledge and research advancement, Philos. T. Roy. Soc. B, 362, 997–1008, https://doi.org/10.1098/rstb.2007.2029, 2007.
Keeler, B. L., Hobbie, S. E., and Kellogg, L. E.: Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition, Ecosystems, 12, 1–15, https://doi.org/10.1007/s10021-008-9199-z, 2009.
Li, X. G., Zhang, M. L., Li, Z. T., Shi X. M., Ma, Q., and Long, R. J.: Dynamics of soil properties and organic carbon pool in topsoil of zokor-made mounds at an alpine site of the Qinghai-Tibetan Plateau, Biol. Fert. Soils, 45, 865–872, https://doi.org/10.1007/s00374-009-0398-3, 2009.
Liang, D. F., Niu, K. C., and Zhang, S. T.: Interacting effects of yak dung deposition and litter quality on litter mass loss and nitrogen dynamics in Tibetan alpine grassland, Grass Forage Sci., 73, 123–131, https://doi.org/10.1111/gfs.12280, 2018.
Luo, C. Y., Xu, G. P, Chao, Z. G., Wang, S. P., Lin, X. W., Hu, Y. G., Zhang, Z. H., Duan, J. C., Chang, X. F., Su, A. L., Li, Y. N., Zhao, X. Q., Du, M. Y., Tang, Y. H., and Bruce, K.: Effect of warming and grazing on litter mass loss and temperature sensitivity of litter and dung mass loss on the Tibetan plateau, Glob. Change Biol., 16, 1606–1617, https://doi.org/10.1111/j.1365-2486.2009.02026.x, 2010.
Ma, Y. S., Zhang, Z. H., Dong, Q. M., Shi, J. J., Wang, Y. L., and Sheng, L.: Application of restoration ecology in “black soil type” degraded grassland rebuilding, Journal of Gansu Agricultural University, 42, 91–97, https://doi.org/10.13432/j.cnki.jgsau.2007.02.021, 2007.
Manzoni, S., Jackson, R. B., Trofymow, J. A., and Porporato, A.: The global stoichiometry of litter nitrogen mineralization, Science, 321, 684, https://doi.org/10.1126/science.1159792, 2008.
McCurdy, J. D., Mcelroy, J. S., Guertal, E. A., and Wood, C. W.: Dynamics of white clover decomposition in a southeastern Bermudagrass lawn, Agron. J., 105, 1277–1282, https://doi.org/10.2134/agronj2013.0058, 2013.
Moretto, A. S., Distel, R. A., and Didoné, N. G.: Decomposition and nutrient dynamic of leaf litter and roots from palatable and unpalatable grasses in a semi-arid grassland, Appl. Soil Ecol., 18, 31–37, https://doi.org/10.1016/S0929-1393(01)00151-2, 2001.
Nelson, D. and Sommers, L.: Total carbon, organic carbon and organic matter, in: Methods of soil analysis. Part 3: chemical methods, edited by: Sparks, D. L., Page, A. L., Helmke, P. A., Loeppert, R. H., Soltanpour, P. N., Tabatabai, M. A., Johnston, C. T., and Sumner, M. E., Soil Science Society of America Book Series, Number 5, Soil Science Society of America, Wisconsin, 961–1010 pp., 1996.
Niu, K. C., Choler, P., Zhao, B. B., and Du, G. Z.: The allometry of reproductive biomass in response to land use in Tibetan alpine grasslands, Funct. Ecol., 23, 274–283, https://doi.org/10.1111/j.1365-2435.2008.01502.x, 2009.
Niu, K. C., Zhang, S. T., Zhao, B. B., and Du, G. Z.: Linking grazing response of species abundance to functional traits in the Tibetan alpine meadow, Plant Soil, 330, 215–223, https://doi.org/10.1007/s11104-009-0194-8, 2010.
Olofsson, J. and Oksanen, L.: Role of litter decomposition for the increased primary production in areas heavily grazed by reindeer: a litterbag experiment, Oikos, 96, 507–515, https://doi.org/10.1034/j.1600-0706.2002.960312.x, 2002.
Orsborne, J. L. and Macauley, B. J.: Decomposition of Eucalyptus leaf litter: influence of seasonal variation in temperature and moisture conditions, Soil Biol. Biochem., 20, 369–375, https://doi.org/10.1016/0038-0717(88)90018-1, 1988.
Pavlů, V., Hejcman, M., Pavlanduring, L., and Gaisler, J.: Restoration of grazing management and its effect on vegetation in an upland grassland, Appl. Veg. Sci., 10, 375–382, https://doi.org/10.1111/j.1654-109X.2007.tb00436.x, 2008.
Ritchie, M. E. and Knops, J. M. H.: Herbivore effects on plant and nitrogen dynamics in oak savannah, Ecology, 79, 165–177, https://doi.org/10.1890/0012-9658(1998)079[0165:HEOPAN]2.0.CO;2, 1998.
Semmartin, M., Aguiar, M. R., Distel, R. A., Moretto, A. S., and Ghersa, C. M.: Litter quality and nutrient cycling affected by grazinginduced species replacements along a precipitation gradient, Oikos, 107, 148–160, https://doi.org/10.1111/j.0030-1299.2004.13153.x, 2004.
Semmartin, M., Garibaldi, L. A., and Chaneton, E. J.: Grazing history effects on above-and below-ground litter decomposition and nutrient cycling in two co-occurring grasses, Plant Soil, 303, 177–189, https://doi.org/10.1007/s11104-007-9497-9, 2008.
Silver, W. L. and Ryan, K. M.: Global patterns in root decomposition: comparisons of climate and litter quality effects, Oecologia, 129, 407–419, https://doi.org/10.1007/s004420100740, 2001.
Sirotnak, J. M. and Huntly, N. J.: Direct and indirect effects of herbivores on nitrogen dynamics: voles in riparian areas, Ecology, 81, 78–87, https://doi.org/10.1890/0012-9658(2000)081[0078:DAIEOH]2.0.CO;2, 2000.
Sternberg, M., Gutman, M., Perevolotsky, A., Ungar, E. D., and Kigel, J.: Vegetation response to grazing management in a Mediterranean herbaceous community: a functional group approach, J. Appl. Ecol., 37, 224–237, https://doi.org/10.1046/j.1365-2664.2000.00491.x, 2000.
Strickland, M. S., Osburn, E., Lauber, C., Fierer, N., and Bradford, M. A.: Litter quality is in the eye of the beholder: initial decomposition rates as a function of inoculum characteristics, Funct. Ecol., 23, 627–636, https://doi.org/10.1111/j.1365-2435.2008.01515.x, 2009.
Sun, D. S., Wesche, K., Chen, D. D., Zhang, S. H., Wu, G. L., Du, G. Z., and Comerford, N. B.: Grazing depresses soil carbon storage through changing plant biomass and composition in a Tibetan alpine meadow, Plant Soil Environ., 57, 271–278, https://doi.org/10.17221/7/2011-PSE, 2011.
Sun, Y., Angerer, J. P., and Hou, F. J.: Effects of grazing systems on herbage mass and liveweight gain of Tibetan sheep in Eastern Qinghai-Tibetan Plateau, China, Rangeland J., 37, 181–190, https://doi.org/10.1071/RJ14062, 2015.
Swift, M. J., Heal, O. W., and Anderson, J. M.: Decomposition in terrestrial ecosystems, Blackwell, Cambridge, Mass., 1979.
Takar, A. A., Dobrowolski, J. P., and Thurow, T. L.: Influence of grazing, vegetation life-form, and soil type on infiltration rates and interrill erosion on a Somalion [Somalian] rangeland, J. Range. Manage., 43, 486–490, https://doi.org/10.2307/4002350, 1990.
Vaieretti, M. V., Cingolani, A. M., Harguindeguy, N. P., and Cabido, M.: Effects of differential grazing on decomposition rate and nitrogen availability in a productive mountain grassland, Plant Soil, 371, 675–691, https://doi.org/10.1007/s11104-013-1831-9, 2013.
van Soest, P. J., Robertson, J. B., and Lewis, B. A.: Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition, J. Dairy Sci., 74, 3583–3597, https://doi.org/10.3168/jds.S0022-0302(91)78551-2, 1991.
Wardle, D., Bonner, K., and Barker, G.: Linkages between plant litter decomposition, litter quality, and vegetation responses to herbivores, Funct. Ecol., 16, 585–595, https://doi.org/10.1046/j.1365-2435.2002.00659.x, 2002.
Wen, L., Dong, S. K., Zhu, L., Li, X. Y., Shi, J. J., Wang, Y. L., and Ma, Y. S.: The construction of grassland degradation index for alpine meadow in Qinghai-Tibetan Plateau, Procedia Environ. Sci., 2, 1966–1969, https://doi.org/10.1016/j.proenv.2010.10.210, 2010.
Wu, G. L., Du, G. Z., Liu, Z. H., and Thirgood, S.: Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai-Tibetan Plateau, Plant Soil, 319, 115–126, https://doi.org/10.1007/s11104-008-9854-3, 2009.
Wu, G. L., Liu, Z. H., Zhang, L., and Chen, J. M.: Long-term fencing improved soil properties and soil organic carbon storage in an alpine swamp meadow of western China, Plant Soil, 332, 331–337, https://doi.org/10.1007/s11104-010-0299-0, 2010.
Zhang, D. Q., Hui, D. F., Luo, Y. Q., and Zhou, G. Y.: Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors, J. Plant Ecol., 1, 85–93, https://doi.org/10.1093/jpe/rtn002, 2008.
Zhu, W. Y., Wang, J. Z., Zhang, Z. H., Ren, F., Chen, L. T., and He, J. S.: Changes in litter quality induced by nutrient addition alter litter decomposition in an alpine meadow on the Qinghai-Tibet Plateau, Sci. Rep. UK, 6, 34290, https://doi.org/10.1038/srep34290, 2016.